A method for improving nonlinear absorption properties of twisted acene compounds
A technology for nonlinear absorption and benzene compounds, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of aminohydroxyl compounds. The effect of absorbing properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
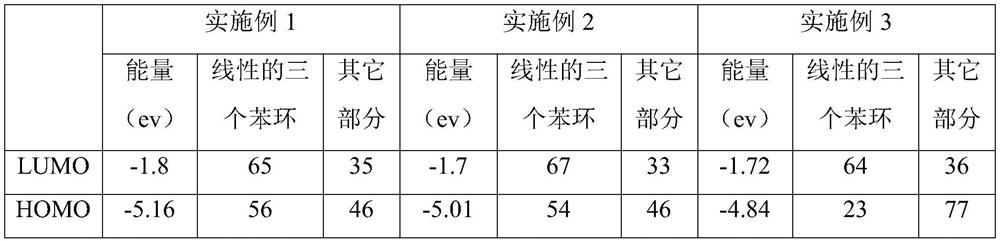
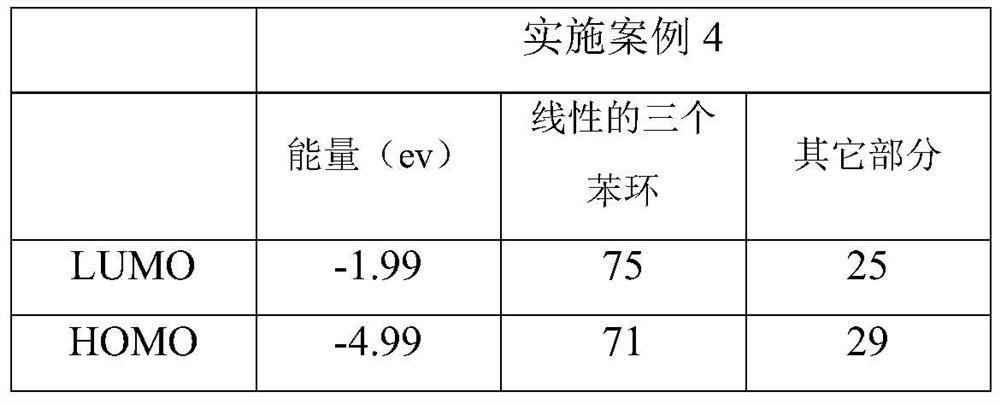
[0007] Specific Embodiment 1: This embodiment is a method for improving the nonlinear absorption performance of twisted acene compounds. The twisted acene compound is a pyrenylacene molecule, and its structural formula is: Improve the ratio of the LUMO orbital occupancy of the three benzene ring structures of the linear pyrenyl acene molecule to the occupancy ratio of the HOMO orbital, that is, the ratio of LUMO / HOMO, and the linear three benzene ring structures of the pyrenyl acene molecule are divided by linear The structure of three benzene rings outside the pyrenyl benzene ring at the end of naphthacene.
[0008] There are large conjugated systems in the molecules of twisted acene compounds, not all conjugated systems in the molecules play a decisive role in the nonlinear absorption performance, such as pyrenyl acene molecules, its optical nonlinear function The center is a linear three benzene ring structure (three benzene ring structures except the pyrenyl benzene ring ...
specific Embodiment approach 2
[0009] Embodiment 2: The difference between this embodiment and Embodiment 1 is that the pyrenyl acene molecule is 2,7-di-tert-butyl-9,17-diphenyl-11-methoxy-12 -((4-fluorophenyl)ethynyl)-dibenzo[de,qr]tetracene, the structural formula of which is: The LUMO orbital occupancy rate is 65%, the HOMO orbital occupancy rate is 56%, and the ratio of LUMO / HOMO is 1.16; the two-photon absorption cross section at the wavelength of 515nm is 561GM. Others are the same as the first embodiment.
[0010] Due to the 2,7-di-tert-butyl-9,17-diphenyl-11-methoxy-12-((4-fluorophenyl)ethynyl)-dibenzo[de,qr obtained in this embodiment ]Tetracene has a two-photon absorption cross section of 561GM at a wavelength of 515nm, so it can be used to prepare optical limiting devices.
specific Embodiment approach 3
[0011] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the 2,7-di-tert-butyl-9,17-diphenyl-11-methoxy-12-((4-fluoro The synthetic method of phenyl) ethynyl)-dibenzo [de, qr] tetracene is as follows:
[0012] Put 2,7-di-tert-butyl-9,17-diphenyl-11-methoxy-12-bromo-dibenzo[de,qr]tetracene into the reactor, add tetrahydrofuran and triethyl amine, then add 4-fluorophenylacetylene, add cuprous iodide, triphenylphosphine and bistriphenylphosphine palladium dichloride under the condition of nitrogen protection, then heat up to 80°C, and react at a temperature of 80°C 22h to 26h, then cooled to room temperature, added saturated brine, and extracted with dichloromethane, collected the organic phase, evaporated to dryness under reduced pressure, separated and purified by silica gel chromatography, the eluent was petroleum ether / dichloromethane mixture, The petroleum ether / dichloromethane mixture is formed by mixing petroleum ether and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com