Starburst molecule containing 2,4,6-tri(2-thineyl)-1,3,5-s-triazine unit and preparation method thereof and use
A s-triazine, thienyl-based technology, applied in the field of star-shaped compounds, can solve the problems of synthesis and performance that few people have explored, and achieve the effects of excellent luminescence performance and good thermal stability.
Inactive Publication Date: 2010-12-22
WUHAN UNIV
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
At present, the research on small molecules and polymer materials with triphenyl-s-triazine as the core is more common, but few people have explored the synthesis and performance of organic (polymer) photoelectric functional materials with aromatic heterocyclic s-triazine as the core. Research
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Login to View More
Abstract
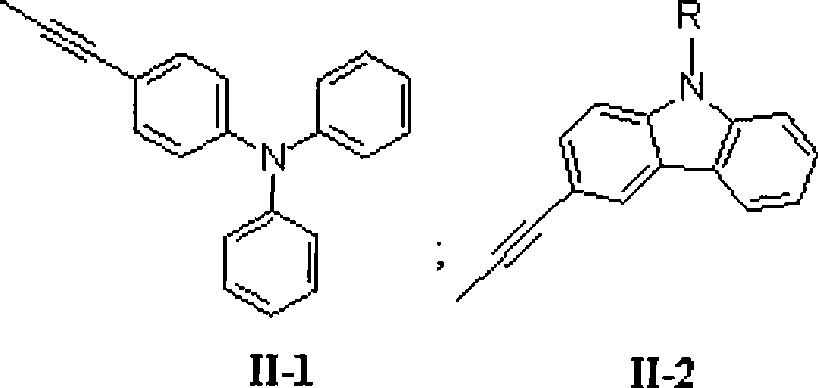
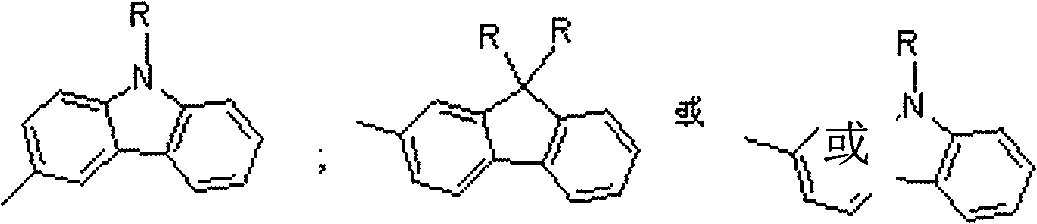
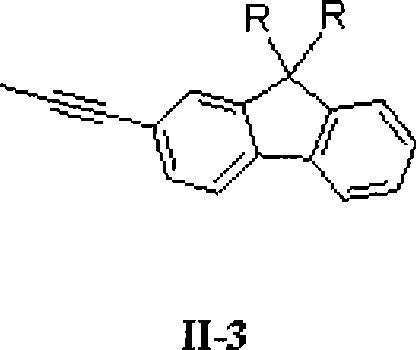
The invention relates to a stellated compound which contains 2, 4, 6-tri [2-thienyl)-1, 3, 5-sym-triazine units, wherein the structural formula of the stellated compound is as shown in a figure. The preparation method for the stellated compound is as follows: 2, 4, 6-tri [2-(5- bromine thienyl)]-1,3,5-sym-triazine prepared by bromination reaction of the 2, 4, 6-tri [2- thienyl)-1, 3, 5-sym-triazine undergoes Suzuki reaction with mono-bromination arene ArBr under the catalysis of Ed(PPh3)4, so as to obtain the stellated compound of the 2, 4, 6-tri (2- thienyl)-1, 3, 5-sym-triazine units; or the 2,4,6-tri [2-(5- bromine thienyl)]-1,3,5-sym-triazine and mono-acetylenyl-substituted aromatic ArH undergo Sonogashira reaction under the catalysis of the Pd(PPh3)4 and CuI, so as to obtain the stellated compound which contains the 2, 4, 6-tri (2- thienyl)-1, 3, 5-sym-triazine units. The stellated compound which contains the 2, 4, 6-tri (2- thienyl)-1, 3, 5-sym-triazine units is applied to the field of organic luminescent materials or organic two-photon absorption materials.
Description
technical field The invention relates to a star compound containing 2,4,6-tris(2-thienyl)-1,3,5-s-triazine unit and its preparation method and application. technical background Due to its unique structural characteristics and excellent chemical and thermal stability, s-triazine molecules and their derivatives have been widely used in the research fields of organic optoelectronic functional materials such as electron injection and transport, nonlinear optics, electroluminescence and supramolecular construction. received widespread attention. At present, the research on small molecules and polymer materials with triphenyl-s-triazine as the core is more common, but few people have explored the synthesis and performance of organic (polymer) photoelectric functional materials with aromatic heterocyclic s-triazine as the core. Research. Since organic (polymer) compounds containing thiophene units exhibit unique photoelectric properties in the field of optoelectronic functional m...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D409/14C09K11/06
Inventor 陈兴国邹丽秦金贵
Owner WUHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com