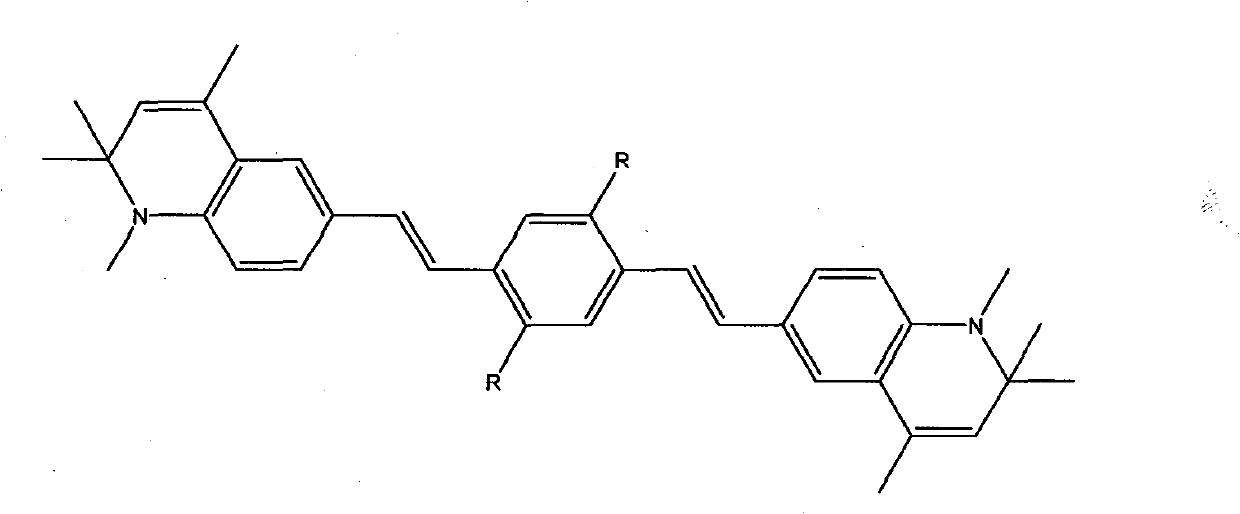
Two-photon induced luminous compound with symmetrical both ends and synthesis method and purposes thereof
An induced luminescence, two-photon technology, applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., can solve the problem of reducing the luminous efficiency of compounds, and achieve the effect of simple doping method, high transparency, and overcoming the easy occurrence of embrittlement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] To dissolve 2.96 g (7.2 mmol) of 2,5-dimethoxy-1,4-bis(diethoxyphosphoryl)methyl-benzene in 50 ml of redistilled N,N-dimethylformamide ( DMF) solution, add 2.38 grams (44.0mmol) sodium methylate, after stirring for 20 minutes, add 3.44 grams (16.0mmol) 1,2,2,4-tetramethyl-1,2-dihydro-6-formylquinone Phenyl, react at room temperature for 1.5 hours, then pour the product solution into water and filter. The filter residue was filtered with hot ethanol below the boiling point, and then separated by silica gel column chromatography (eluent: dichloromethane / cyclohexane=100 / 20, v / v), the solvent was removed by rotary evaporation, and the pure product was obtained by vacuum drying. The present invention uses 1,2,2,4-tetramethyl-1,2-dihydro-6-formyl quinoline and 2,5-dimethoxy-1,4-bis(diethoxyphosphoryl ) methyl-benzene as a raw material, the prepared 1,2,2,4-tetramethyldihydroquinoline compound with two-photon induced luminescence characteristic of two-photon symmetry. M.P.23...
Embodiment 2
[0028] Add 2.51 g (46.4 mmol) of sodium methoxide to 2.60 g (7.4 mmol) of 1,4-bis(diethoxyphosphoryl)methyl-benzene in 50 ml of redistilled DMF solution, stir for 25 minutes, then add 3.70 g (17.2 mmol) of 1,2,2,4-tetramethyl-1,2-dihydro-6-formylquinoline were reacted at room temperature for 3 hours, and then the product solution was poured into water and filtered. The filter residue was filtered with hot ethanol below the boiling point, and then separated by silica gel column chromatography (eluent: dichloromethane / cyclohexane=100 / 20, v / v), the solvent was removed by rotary evaporation, and the pure product was obtained by vacuum drying. The present invention uses 1,2,2,4-tetramethyl-1,2-dihydro-6-formyl quinoline and 1,4-bis(diethoxyphosphoryl)methyl-benzene as raw materials to prepare 1,2,2,4-Tetramethyldihydroquinoline compounds with two-photon-induced luminescence characteristics of two-terminal symmetry. 1HNMR (300MHz, CDCl3): δ7.51(s, 4H), 7.33-6.89(m, 8H), 6.53(d, 2H,...
Embodiment 3
[0031] To a solution of 2.95 g (7.8 mmol) of 2,5-dimethyl-1,4-bis(diethoxyphosphoryl)methyl-benzene in 50 mL of redistilled DMF was added 2.72 g (50.4 mmol) of methanol Sodium, after stirring for 30 minutes, add 4.13 grams (19.2mmol) 1,2,2,4-tetramethyl-1,2-dihydro-6-formylquinoline, react at room temperature for 2.0 hours, after that the product solution Pour into water and filter. The filter residue was filtered with hot ethanol below the boiling point, and then separated by silica gel column chromatography (eluent: dichloromethane / cyclohexane=100 / 20, v / v), the solvent was removed by rotary evaporation, and the pure product was obtained by vacuum drying. The present invention uses 1,2,2,4-tetramethyl-1,2-dihydro-6-formyl quinoline and 2,5-dimethyl-1,4-bis(diethoxyphosphoryl) The 1,2,2,4-tetramethyldihydroquinoline compound with two-photon induced luminescence characteristics of two-photon-induced luminescence is produced by using methyl-benzene as a raw material. 1 HNMR (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com