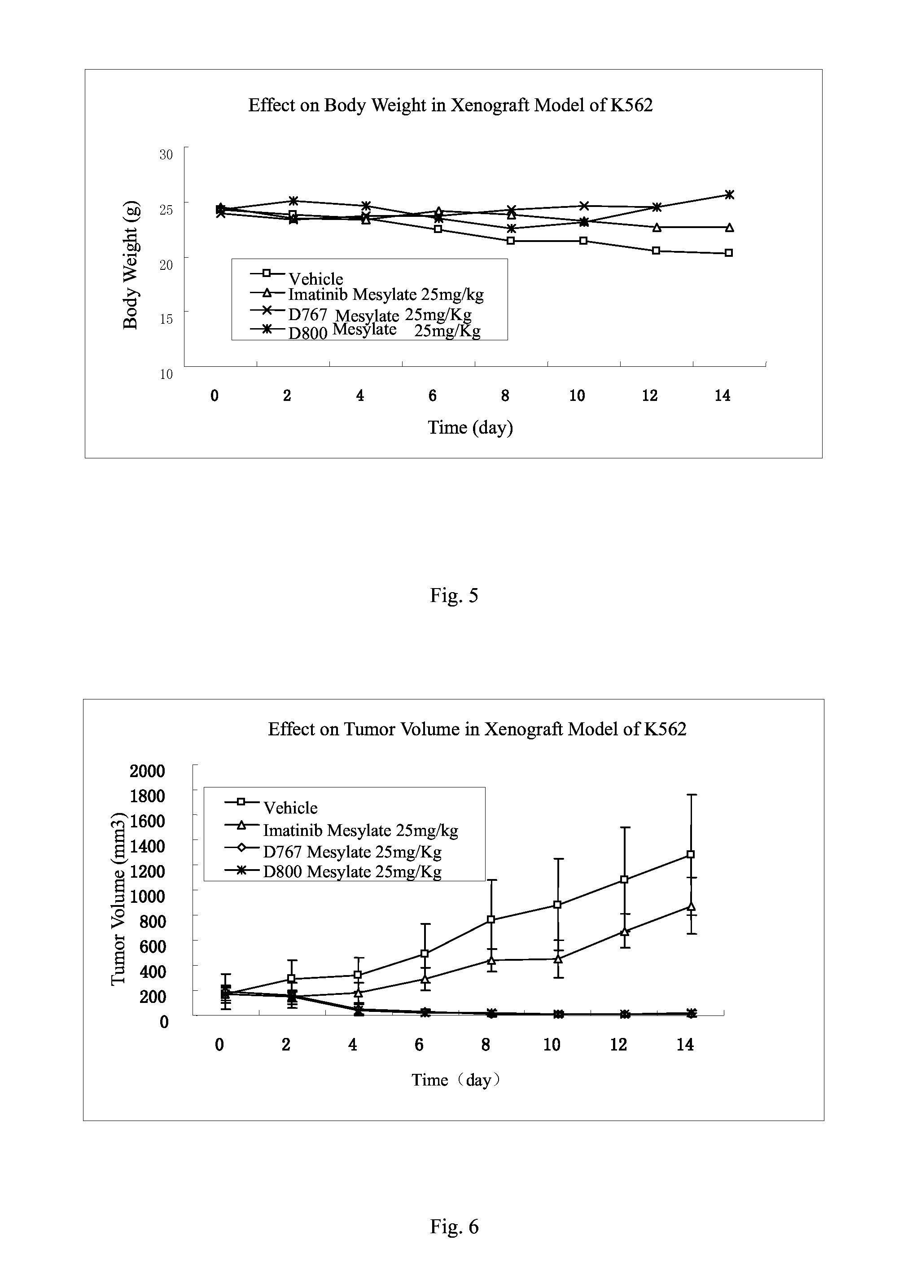
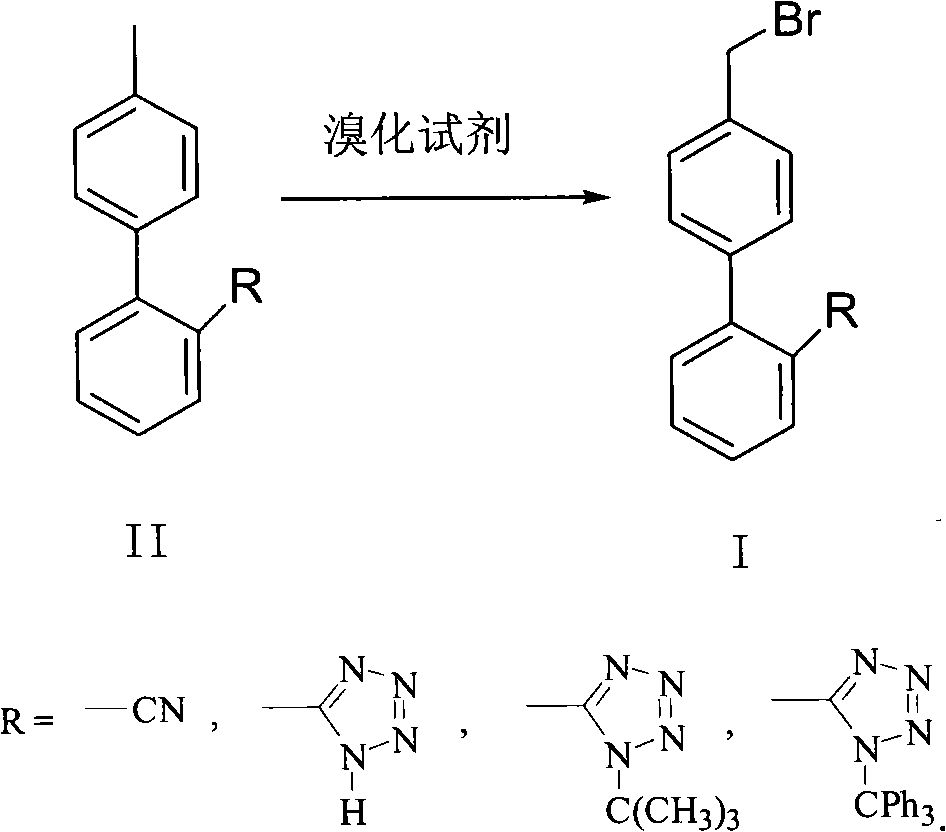
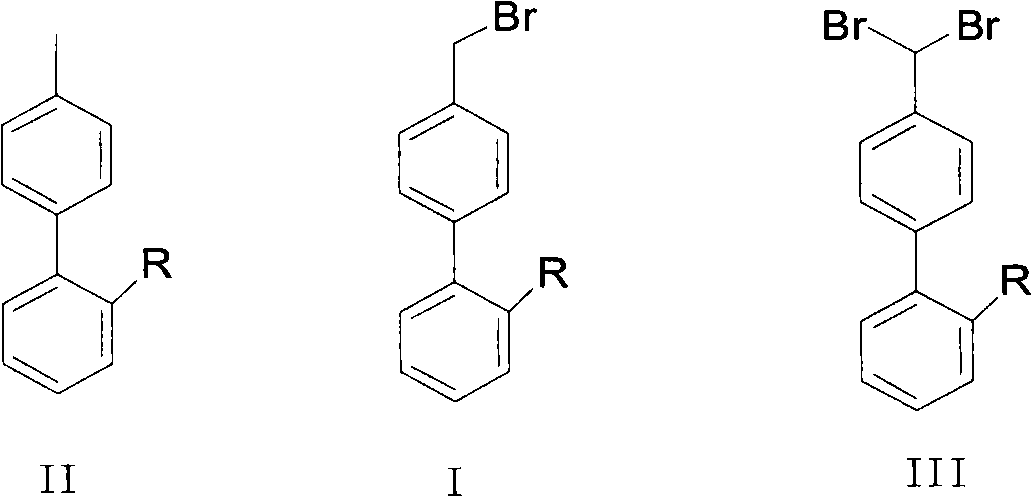
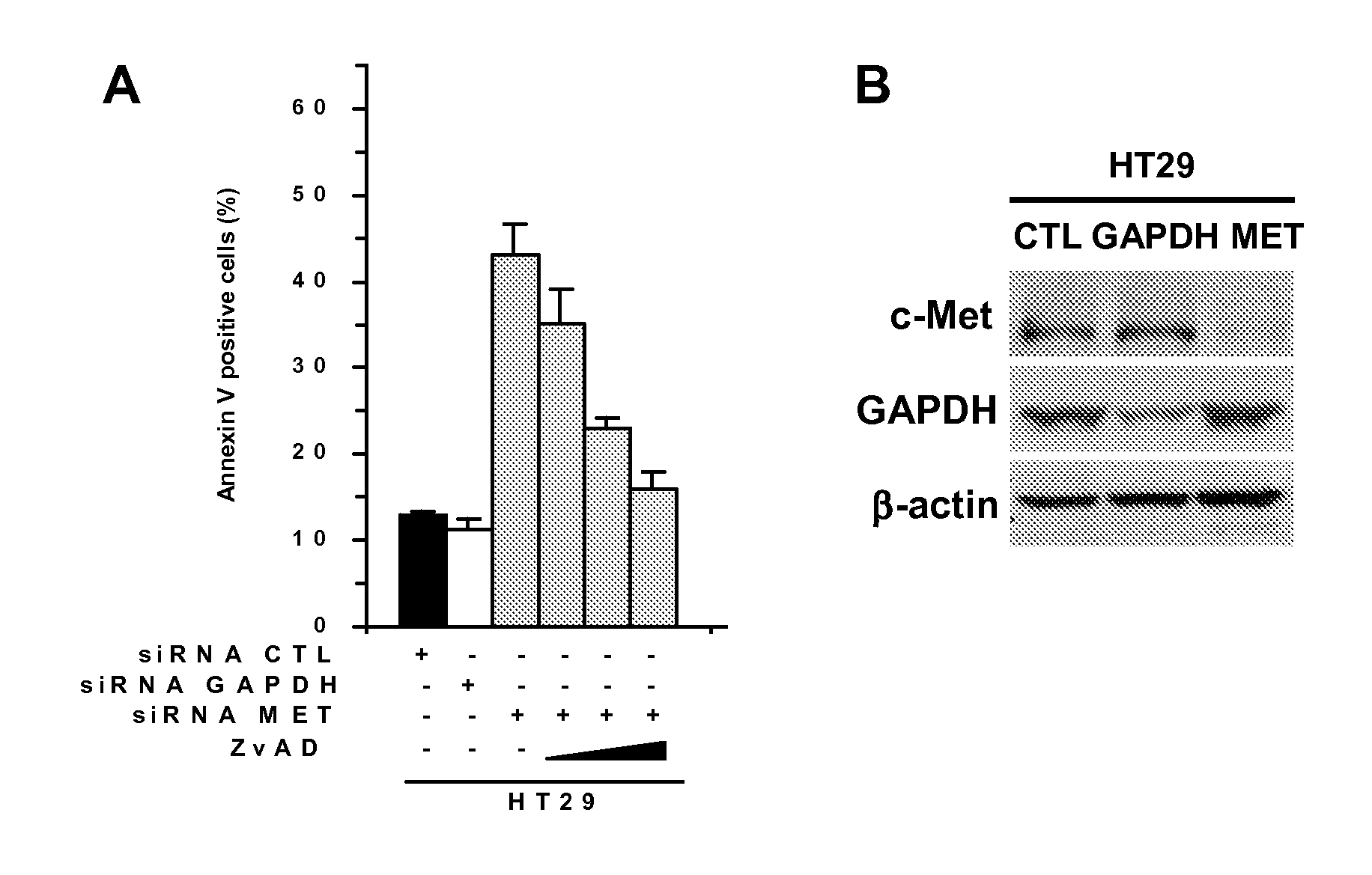
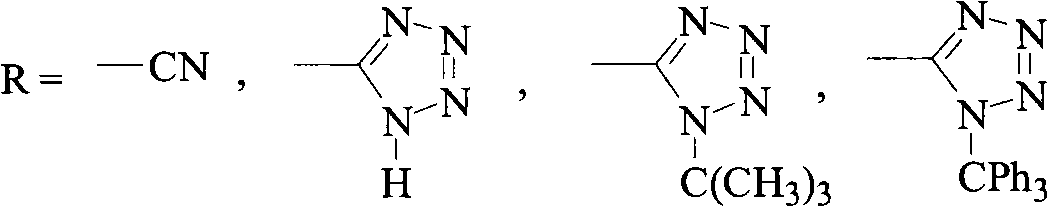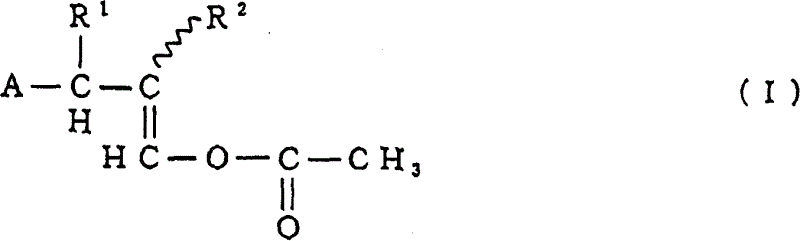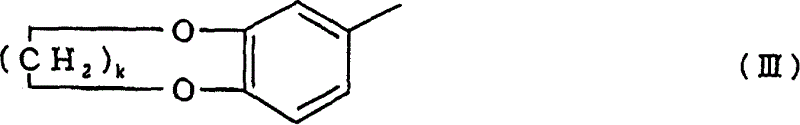Patents
Literature
112 results about "Bephenium Compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Analogs or derivatives of bephenium (N,N-dimethyl-N-(2-phenoxyethyl)benzenemethanaminium).
Aryl- or Heteroaryl-Substituted Benzene Compounds
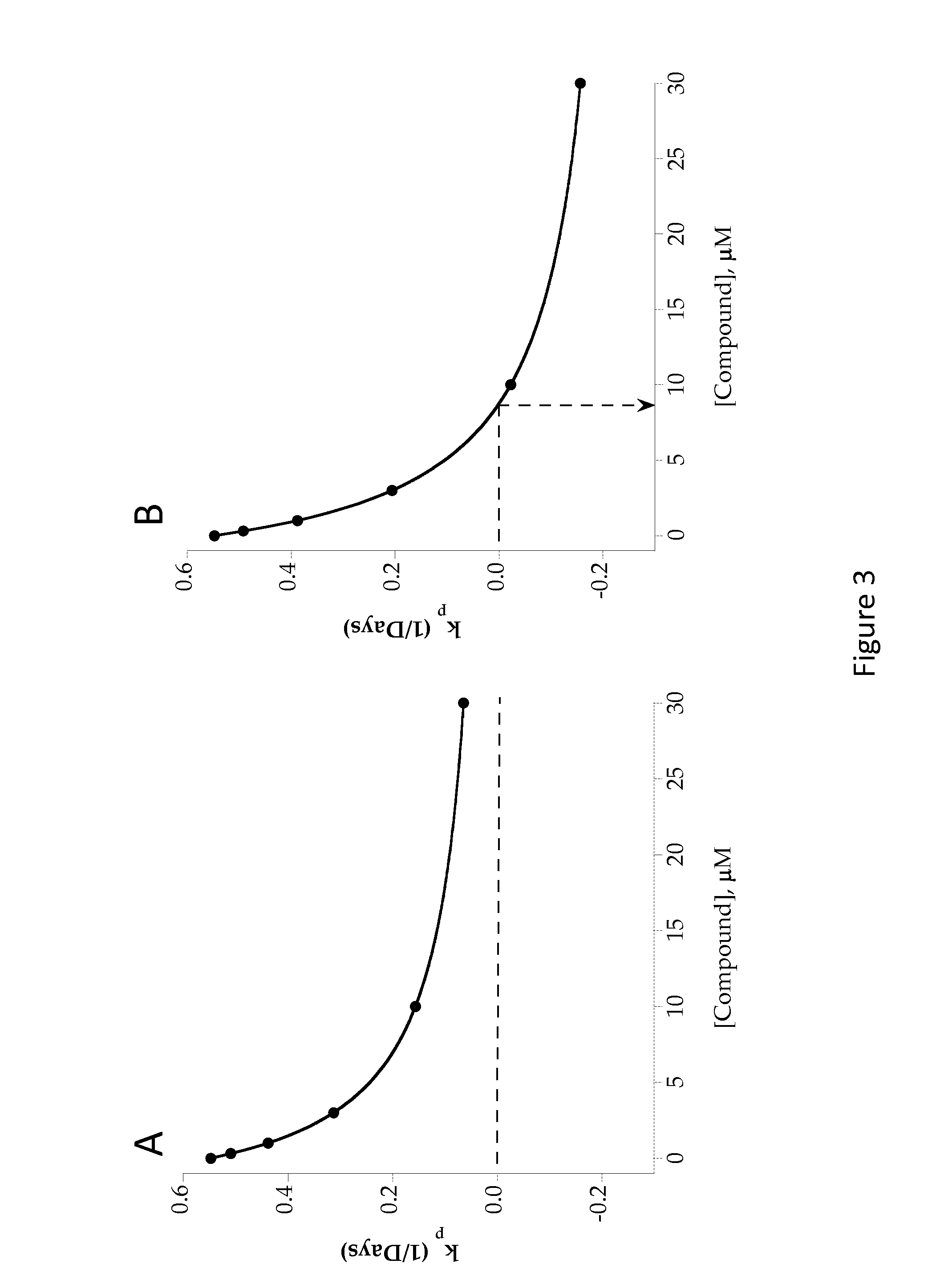
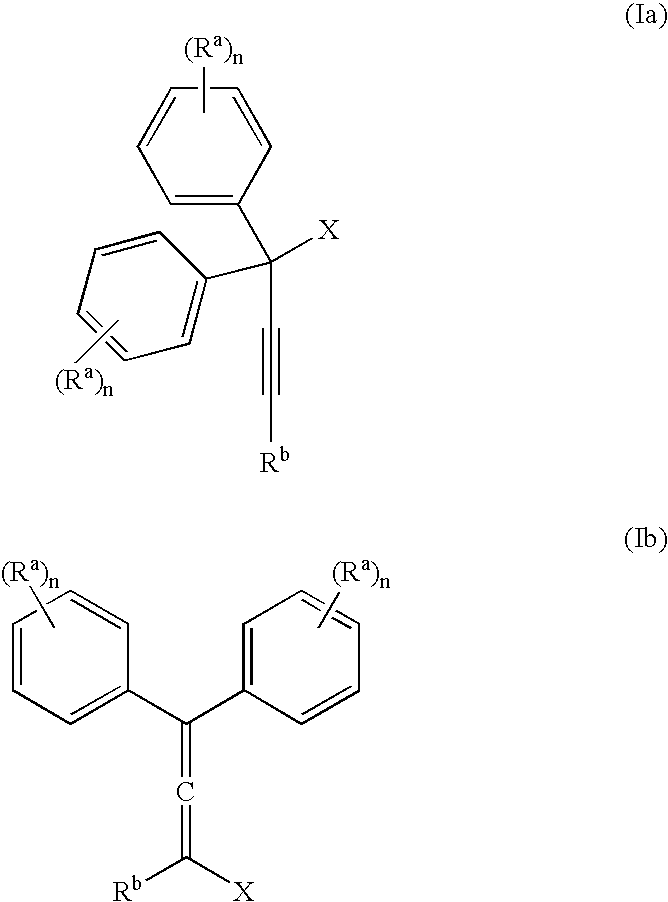
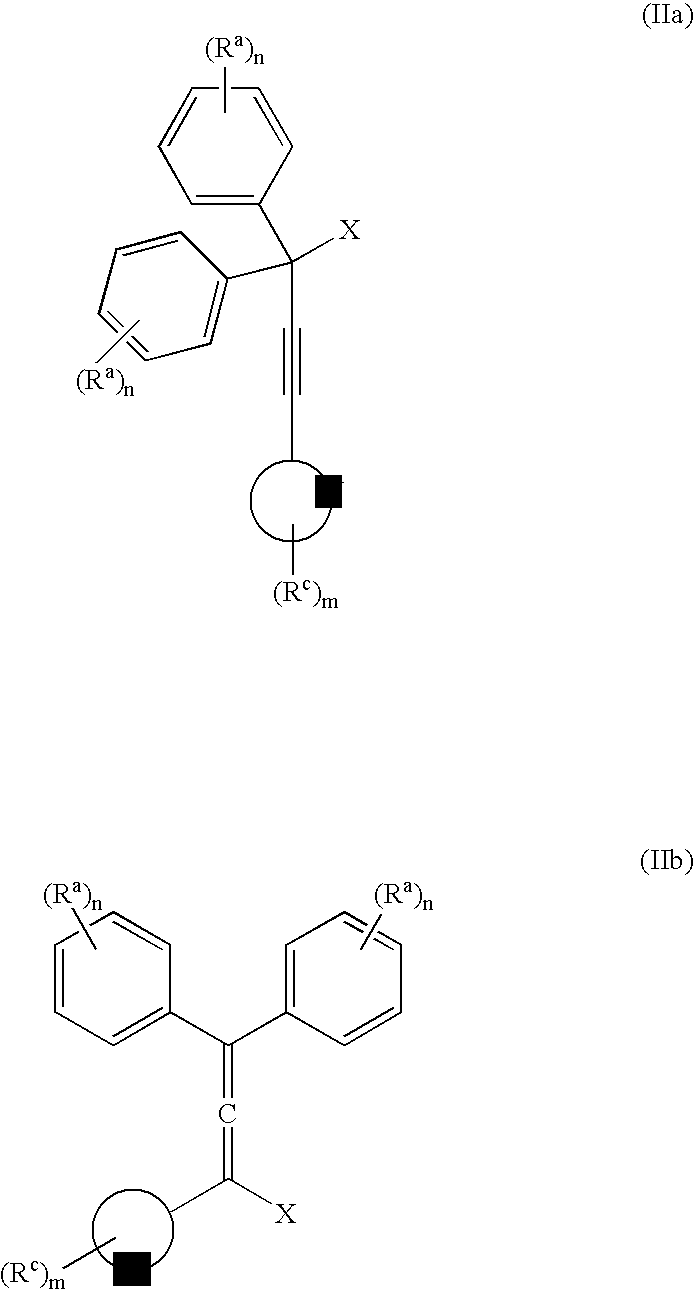
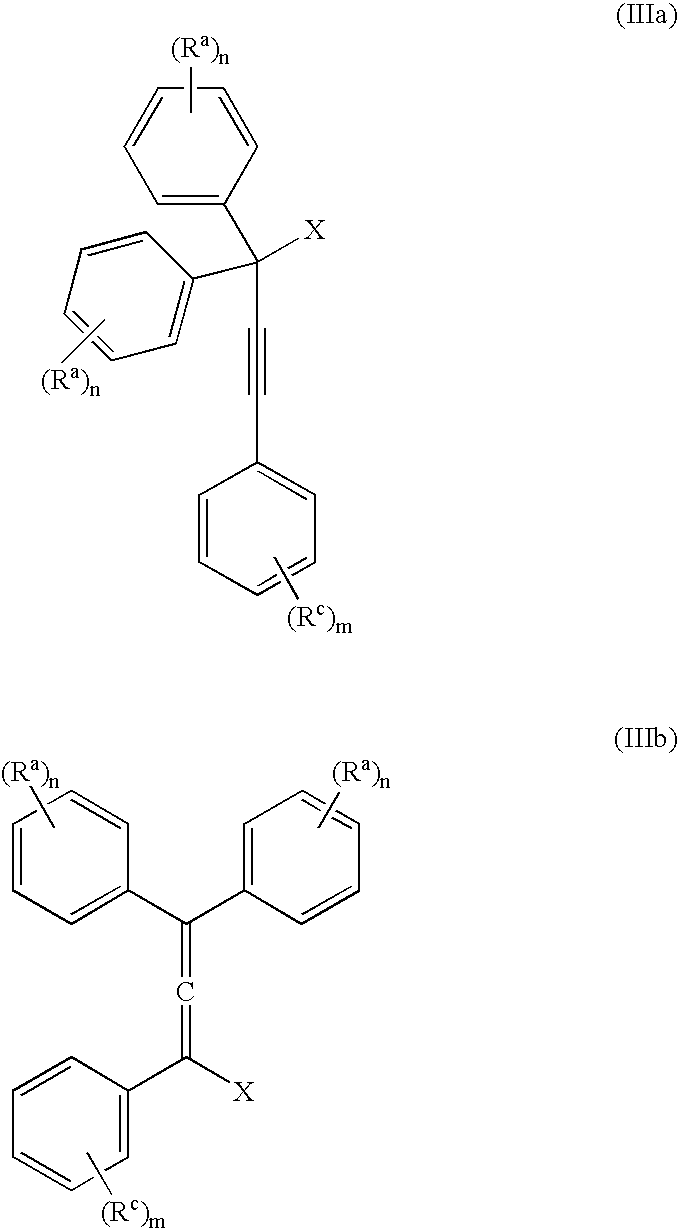
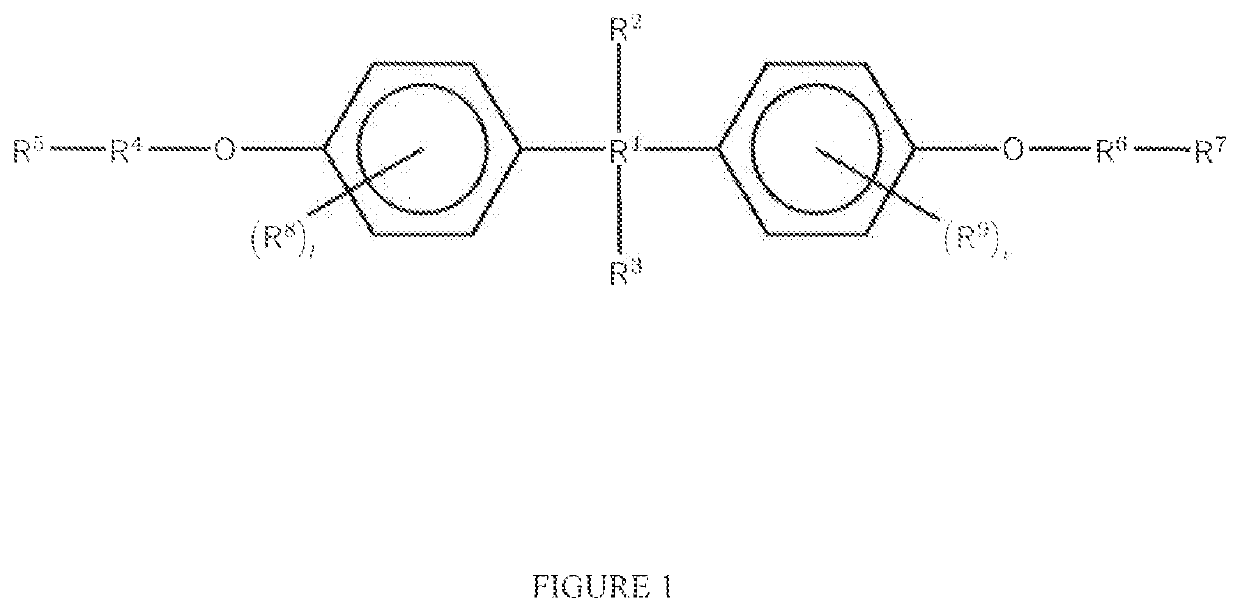
The present invention relates to aryl- or heteroaryl-substituted benzene compounds. The present invention also relates to pharmaceutical compositions containing these compounds and methods of treating cancer by administering these compounds and pharmaceutical compositions to subjects in need thereof. The present invention also relates to the use of such compounds for research or other non-therapeutic purposes.
Owner:EPIZYME
Benzene compounds
InactiveUS20070105899A1Preventing, treating, and arresting the development of these diseasesExcellent ACC inhibiting activityBiocideSenses disorderDiabetic retinopathyDiabetic complication
The present invention provides novel benzene compounds presented by the following formulas, and analogs thereof, that exert an ACC activity-inhibiting effect that is effective in the treatment of obesity, hyperlipemia, fatty liver, hyperglycemia, impaired glucose tolerance, diabetes, diabetic complications (diabetic peripheral neuropathy, diabetic nephropathy, diabetic retinopathy, and diabetic macroangiopathy, hypertension, arteriosclerosis), hypertension, and arteriosclerosis.
Owner:AJINOMOTO CO INC
Substituted Benzene Compounds
The present invention relates to substituted benzene compounds. The present invention also relates to pharmaceutical compositions containing these compounds and methods of treating cancer by administering these compounds and pharmaceutical compositions to subjects in need thereof. The present invention also relates to the use of such compounds for research or other non-therapeutic purposes.
Owner:EPIZYME
Synthesis process
ActiveUS20060025617A1The method is simple and fastHigh yieldOrganic compound preparationHydrocarbon from oxygen organic compoundsAlcoholLeaving group
A process for synthesizing a single isomer of a naphthacene compound comprises the steps of: (a) reacting a symmetrically substituted 1,1-diarylpropargyl alcohol compound with a reagent capable of forming a leaving group to form a reaction mixture containing a intermediate; and then (b) heating the intermediate in the presence of a solvent and in the absence of any oxidizing agent to form a single naphthacene compound.
Owner:GLOBAL OLED TECH
Sulfonylbenzene compounds as anti-inflammatory/analgesic agents
InactiveUS6294558B1Inhibit prostaniod-induced smooth muscle contractionInhibit synthesisBiocideOrganic chemistryArylHydrogen
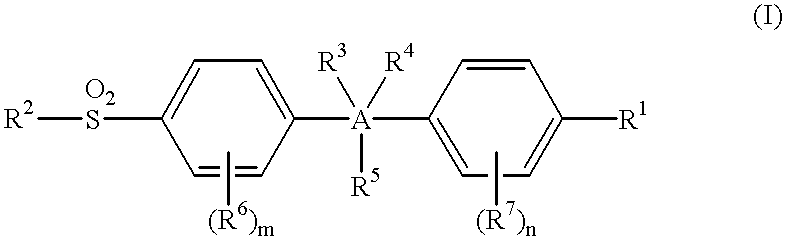
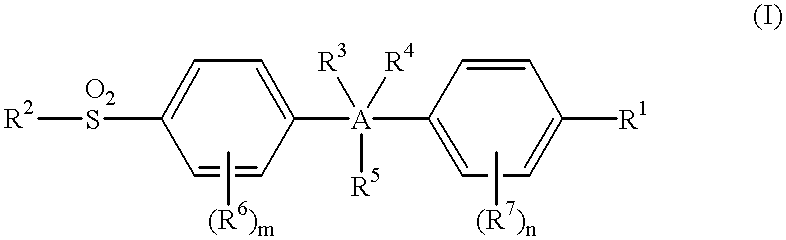
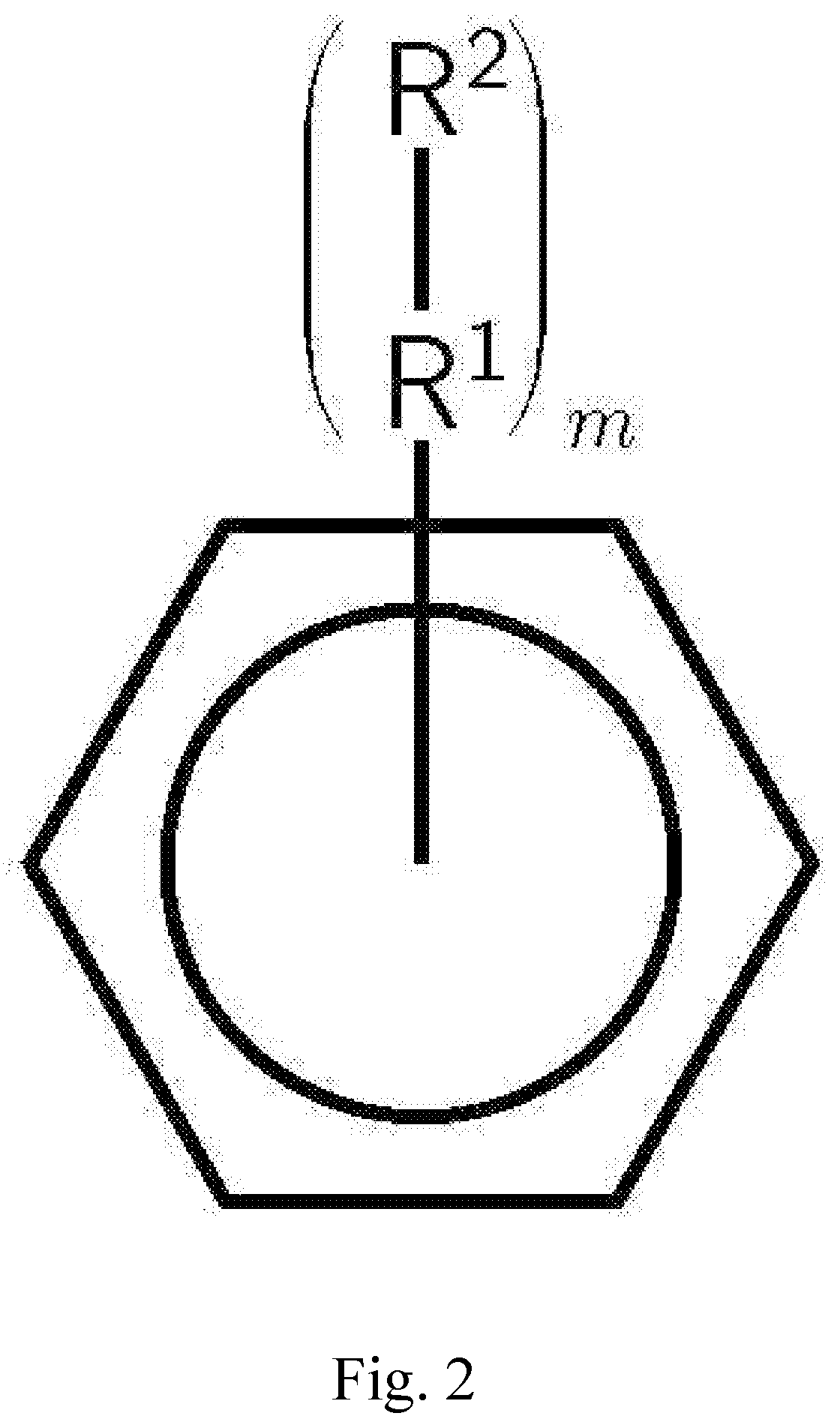
This invention provides a compound of the formula:or its pharmaceutically acceptable salt thereof, wherein A is partially unsaturated or unsaturated five membered heterocyclic, or partially unsaturated or unsaturated five membered carbocyclic, wherein the 4-(sulfonyl)phenyl and the 4-substituted phenyl in the formula (I) are attached to ring atoms of Ring A, which are adjacent to each other; R1 is optionally substituted aryl or heteroaryl, with the proviso that when A is pyrazole, R1 is heteroaryl; R2 is C1-4 alkyl, halo-substituted C1-4 alkyl, C1-4 alkylamino, C1-4 dialkylamino or amino; R3, R4 and R5 are independently hydrogen, halo, C1-4 alkyl, halo-substituted C1-4 alkyl or the like; or two of R3, R4 and R5 are taken together with atoms to which they are attached and form a 4-7 membered ring; R6 and R7 are independently hydrogen, halo, C1-4 alkyl, halo-substituted C1-4 alkyl, C1-4 alkoxy, C1-4 alkylthio, C1-4 alkylamino or N,N-di C1-4 alkylamino; and m and n are independently 1, 2, 3 or 4. This invention also provides a pharmaceutical composition useful for the treatment of a medical condition in which prostaglandins are implicated as pathogens.
Owner:PFIZER INC
Benzene compound and salt thereof
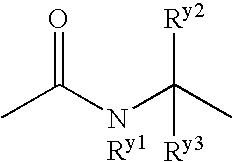
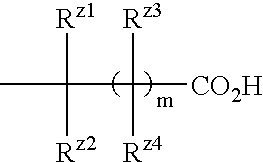
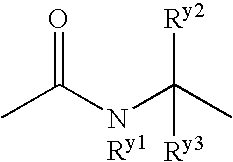
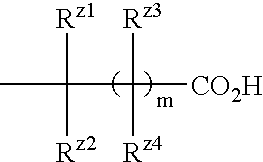
The present invention provides a medicament comprising a benzene compound useful as an insulin sensitizer, a salt thereof or a hydrate of them and a derivative of them as the active ingredient. Specifically, it provides a benzene compound represented by the following formula, a salt thereof or a hydrate of them.In the formula, X represents 1) a C6-10 aryl group which may have one or more substituents or 2) a 5- to 10-membered heteroaryl group which may have one or more substituents; Y represents a group represented by the formula:(in the above formulae, Ry1, Ry2 and Ry3 are the same as or different from one another and each represents a hydrogen atom, a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, or a C3-7 cycloalkyl group) etc.; Z represents a group represented by the formula:(in the formula, m represents an integer of 0 to 2; Rz1, Rz2, Rz3 and Rz4 are the same as or different from one another and each represents a hydrogen atom, a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, a C3-7 cycloalkyl group, a halogenated C1-6 alkyl group, a halogenated C1-6 alkoxy group etc.); and R1, R2, R3 and R4 are the same as or different from one another and each represents a hydrogen atom, a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, a C3-7 cycloalkyl group etc.
Owner:EISIA R&D MANAGEMENT CO LTD
Production method of xylylenediamine
ActiveUS6881864B2Efficient productionSmall contentAmino compound purification/separationOrganic compound preparationNitrileCombinatorial chemistry
In the method of the present invention, xylylenediamine is produced by a two-stage hydrogenation of a dicyanobenzene compound. In a first stage (a), the hydrogenation is performed until a conversion of nitrile groups reaches 90 mol % or higher and less than 99.9 mol %. In a second stage (b), the hydrogenation is further continued at temperatures 10° C. or more higher than in the step (a) until the conversion of nitrile groups reaches a level which is higher than that attained in the step (a) and equal to 99.5 mol % or more. In the present invention, a highly pure xylylenediamine containing a minimized amount of cyanobenzylamine is efficiently produced in a simple manner without needing a specific purification, and also without deteriorating the use efficiency of the catalyst while reducing the amount of the dicyanobenzene compound remaining not reacted and the generation of the intermediate cyanobenzylamine.
Owner:MITSUBISHI GAS CHEM CO INC
Heterocyclic alkynyl benzene compounds and medical compositions and uses thereof
ActiveUS20130196985A1Inhibit massive proliferationOvercome resistanceBiocideOrganic chemistryChemical compositionStereoisomerism
The heterocyclic alkynyl benzene compounds of formula (I), their pharmaceutically acceptable salts and stereoisomers thereof, as well as application in preparing drugs for preventing or treating tumors. The compounds can overcome the clinically induced resistance against Gleevec.
Owner:GUANGZHOU SHUNJIAN PHARM CO LTD
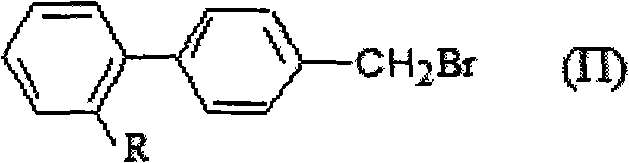
Green synthesis method of bromomethylbiphenyl compound
ActiveCN101648839AHigh selectivityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationBenzeneOrganic solvent
The invention relates to a green synthesis method of a bromomethylbiphenyl compound, comprising the following steps: dissolving a 4'-methyl-2-substituted biphenyl compound in an organic solvent, adding a bromizating agent to carry out bromination reaction and controlling the reaction conditions to obtain the bromomethylbiphenyl compound. The invention is characterized in that the reaction is carried out in light.
Owner:CHINA RESOURCES SAIKE PHARMA
Production method of xylylenediamine
ActiveUS20050004399A1High yieldReduce the amount requiredPreparation by oxidation reactionsAmino compound purification/separationEngineeringCombinatorial chemistry
In the method of the present invention, xylylenediamine is produced by a two-stage hydrogenation of a dicyanobenzene compound. In a first stage (a), the hydrogenation is performed until a conversion of nitrile groups reaches 90 mol % or higher and less than 99.9 mol %. In a second stage (b), the hydrogenation is further continued at temperatures 10° C. or more higher than in the step (a) until the conversion of nitrile groups reaches a level which is higher than that attained in the step (a) and equal to 99.5 mol % or more. In the present invention, a highly pure xylylenediamine containing a minimized amount of cyanobenzylamine is efficiently produced in a simple manner without needing a specific purification, and also without deteriorating the use efficiency of the catalyst while reducing the amount of the dicyanobenzene compound remaining not reacted and the generation of the intermediate cyanobenzylamine.
Owner:MITSUBISHI GAS CHEM CO INC
Benzene compound and salt thereof
InactiveUS20040138271A1Induce experimental colitisGood anti-inflammatory effectBiocideOrganic chemistryPancreatic hormoneBULK ACTIVE INGREDIENT
The present invention provides a medicament comprising a benzene compound useful as an insulin sensitizer, a salt thereof or a hydrate of them and a derivative of them as the active ingredient. Specifically, it provides a benzene compound represented by the following formula, a salt thereof or a hydrate of them. In the formula, X represents 1) a C6-10 aryl group which may have one or more substituents or 2) a 5- to 10-membered heteroaryl group which may have one or more substituents; Y represents a group represented by the formula: (in the above formulae, R<y1>, R<y2 >and R<y3 >are the same as or different from one another and each represents a hydrogen atom, a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, or a C3-7 cycloalkyl group) etc.; Z represents a group represented by the formula: (in the formula, m represents an integer of 0 to 2; R<z1, R><z2>, R<z3 >and R<z4 >are the same as or different from one another and each represents a hydrogen atom, a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, a C3-7 cycloalkyl group, a halogenated C1-6 alkyl group, a halogenated C1-6 alkoxy group etc.); and R<1>, R<2>, R<3 >and R<4 >are the same as or different from one another and each represents a hydrogen atom, a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group, a C3-7 cycloalkyl group etc.
Owner:EISIA R&D MANAGEMENT CO LTD
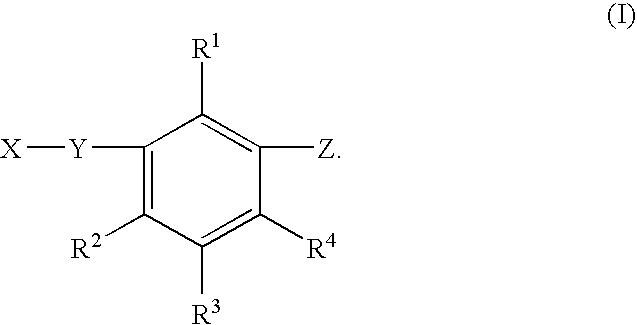
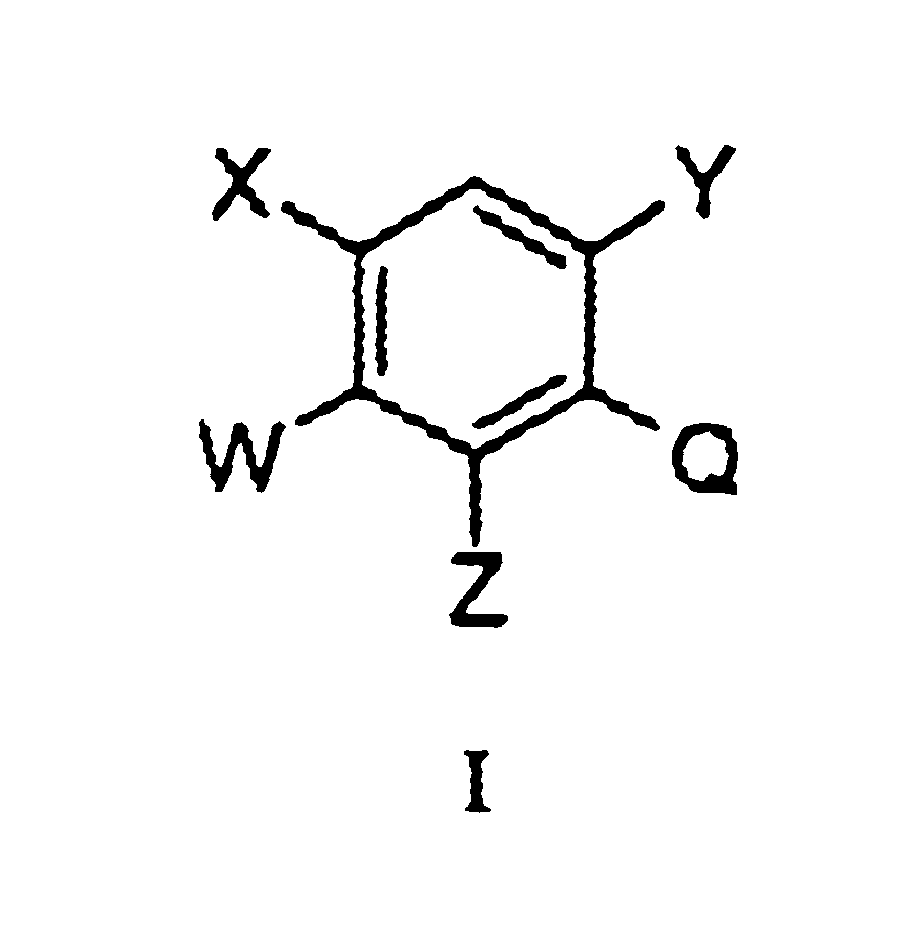
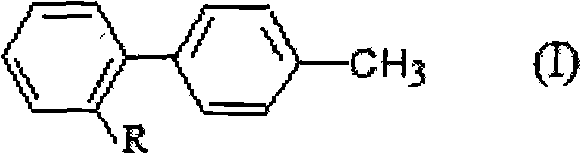
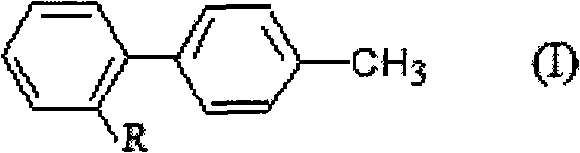
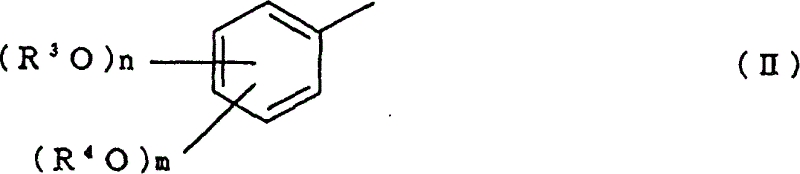
Substituted benzene compounds, process for their preparation and herbicidal and defoliant composition containing them
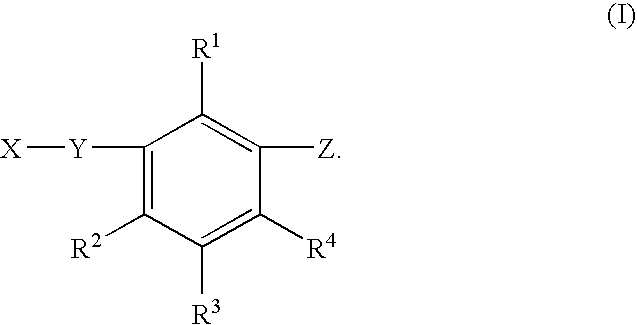
Novel herbicidal and defoliant substituted aniline derived compounds represented by general structure (I) are described. W, X, Y, Z, and Q are as defined in the disclosure. Also described are the processes for the manufacture of these compounds and agriculturally suitable compositions containing these as active ingredients which are useful as herbicides for general or selective pre-emergent or post-emergent control of undesired plant species and defoliants at very low concentrations of these biologically active compounds.
Owner:ISK AMERICAS
Process for producing 1-acetoxy-3-(substituded phenyl) propenes
InactiveUS20060069273A1Improve efficiencyEasy to processOrganic compound preparationPreparation by transesterificationPtru catalystCombinatorial chemistry
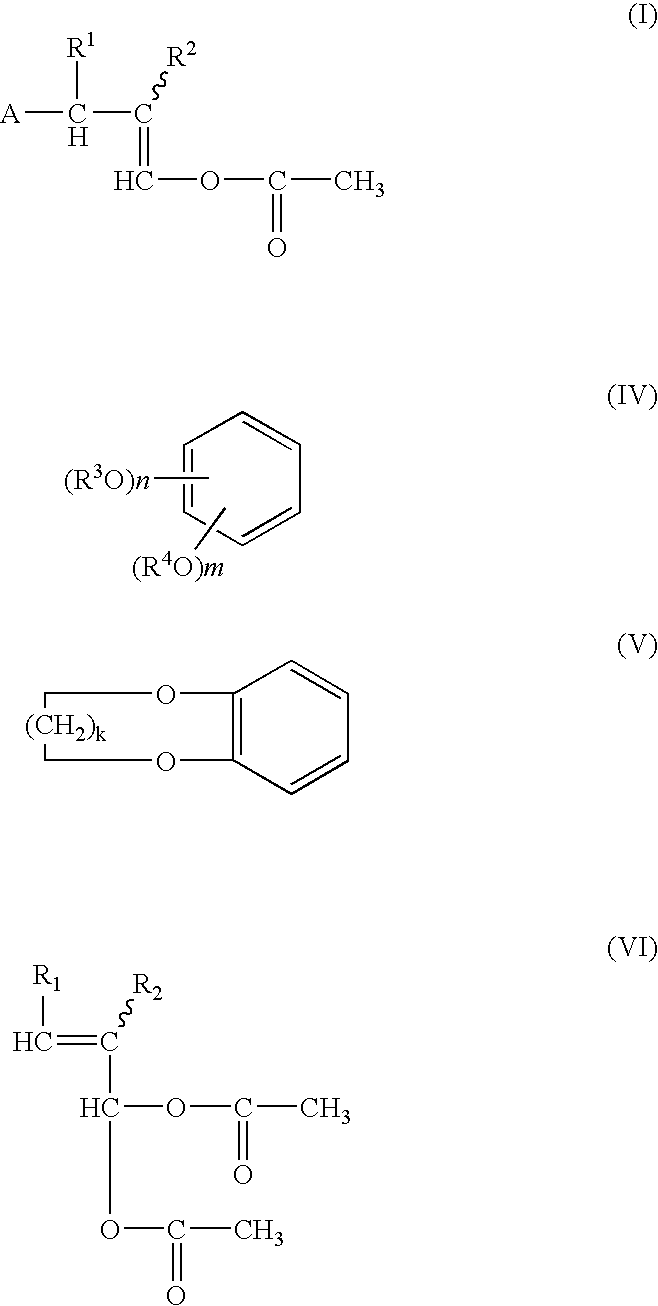
The compounds represented by the formula (I) are produced by reacting benzene compound of the formula (IV) or (V) with alkenylidene diacetate of the formula (VI) in the presence of a catalyst comprising one or more members selected from (a) halogenated boron compounds, (b) triflate compounds of Group 11 elements, (c) halogenated compounds of Group 12 elements, and (d) triflate and halogenated compounds of tin and atomic numbers 58 and 66 to 71 elements. R1, R2=H or C1-C10 alkyl group A=Substituted phenyl group corresponding to a compound of formula (IV) or (V), R3, R4=H or C1-C4 alkyl group, m=0 or 1-4, n=1 to 5, k=1 or 2.
Owner:UBE IND LTD
Process for preparing dialkylbiphenyl isomer mixtures
In a process for producing dialkylbiphenyl compounds, a feed comprising substituted cyclohexylbenzene isomers having the formula (I):wherein each of R1 and R2 is an alkyl group and wherein the feed comprises m % by weight of isomers in which R1 is in the 2-position, based on the total weight of substituted cyclohexylbenzene isomers in the feed; is transalkylated with a compound of formula (II):to produce a transalkylation product comprising substituted cyclohexylbenzene isomers having the formula (I) and including n % by weight of isomers in which R1 is in the 2-position, based on the total weight of substituted cyclohexylbenzene isomers in the transalkylation product, wherein n<m. At least part of the transalkylation product is then dehydrogenated under conditions effective to convert at least part of the substituted cyclohexylbenzene isomers in the transalkylation product to dialkylbiphenyl compounds.
Owner:EXXONMOBIL CHEM PAT INC
Synthesis process
ActiveUS20060025642A1The method is simple and fastHigh yieldOrganic compound preparationHydrocarbon from oxygen organic compoundsLeaving groupSolvent
A process for synthesizing a naphthacene compound comprises the steps of: (a) reacting a propargyl alcohol compound with a reagent capable of forming a leaving group to form a reaction mixture containing an intermediate; and then (b) heating the intermediate in the presence of a solvent and in the absence of any oxidizing agent and in the absence of any base, to form the naphthacene compound.
Owner:GLOBAL OLED TECH
Indolocarbazole tetraphenylene compounds
Indolocarbazole tetraphenylene compounds are disclosed. These novel compounds contain tetraarylene or tetraheteroarylene which can be used as charge transporting materials, charge blocking materials,hosts or emitters in an organic electroluminescent device. These novel compounds offer better device performance than comparative compounds that don't contain a tetraarylene or tetraheteroarylene structure. Also disclosed are an electroluminescent device and a formulation.
Owner:BEIJING SUMMER SPROUT TECH CO LTD
Bromination of hydroxyaromatic compounds and further conversion to dihydroxyaromatic compounds
InactiveCN1756729AOrganic chemistryOrganic compound preparationAlkaline earth metalPhysical chemistry
Brominated hydroxyaromatic compounds such as p-bromophenol are prepared by contacting a hydroxyaromatic compound with oxygen and a bromine source such as hydrogen bromide or an alkali metal or alkaline earth metal bromide in an acidic medium, in the presence of elemental copper or a copper compound as catalyst. The brominated product of this reaction may be converted alternately to a dihydroxyaromatic compound such as hydroquinone by hydrolyses, or a dihydroxybiphenyl compound such as 4,4'-dihydroxybiphenyl by reductive coupling.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Crystalline 2-fluoro-3-nitrotoluene and process for the preparation thereof
PendingUS20210292271A1Easily handableEasily purifiableOrganic compound preparationOrganic chemistry methodsCombinatorial chemistryToluene
Owner:F I S FAB ILTALIANA SINTETICI SPA +1
Method for preparing and recovering aromatic methyl diphenyl compound
ActiveCN101768035AIncrease profitCarboxylic acid nitrile preparationOrganic compound preparationSolventBiphenyl compound
The invention provides a method for preparing and recovering aromatic methyl diphenyl compound (I), wherein, an aromatic methyl diphenyl compound (I) is used for preparing an aromatic bromomethyl diphenyl compound (II), after separating and purifying the product, the aromatic halomethyl diphenyl compound (II) and a compound (III) are contained in a mother liquor, that is: the aromatic methyl diphenyl compound in the mother liquor is reacted with proton donor and active metallic zinc in the solvent and then converted into the aromatic methyl diphenyl compound. By adopting the method, the aromatic halomethyl diphenyl compound in the mother liquor can be converted into the aromatic methyl diphenyl compound for recycle, which has obvious utility value in industrial applications. The aromatic methyl diphenyl compound (I), the aromatic bromomethyl diphenyl compound (II) and the aromatic bibromomethyl diphenyl compound (II) are provided.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Intermediate for heteroacene compound and synthetic method of heteroacene compound using its intermediate
ActiveUS20170117484A1Low costSimple processOrganic chemistryFinal product manufactureChemical formulaCombinatorial chemistry
Owner:SAMSUNG ELECTRONICS CO LTD
Substituted benzene compounds
The present invention relates to substituted benzene compounds. The present invention also relates to pharmaceutical compositions containing these compounds and methods of treating cancer by administering these compounds and pharmaceutical compositions to subjects in need thereof. The present invention also relates to the use of such compounds for research or other non-therapeutic purposes.
Owner:EPIZYME
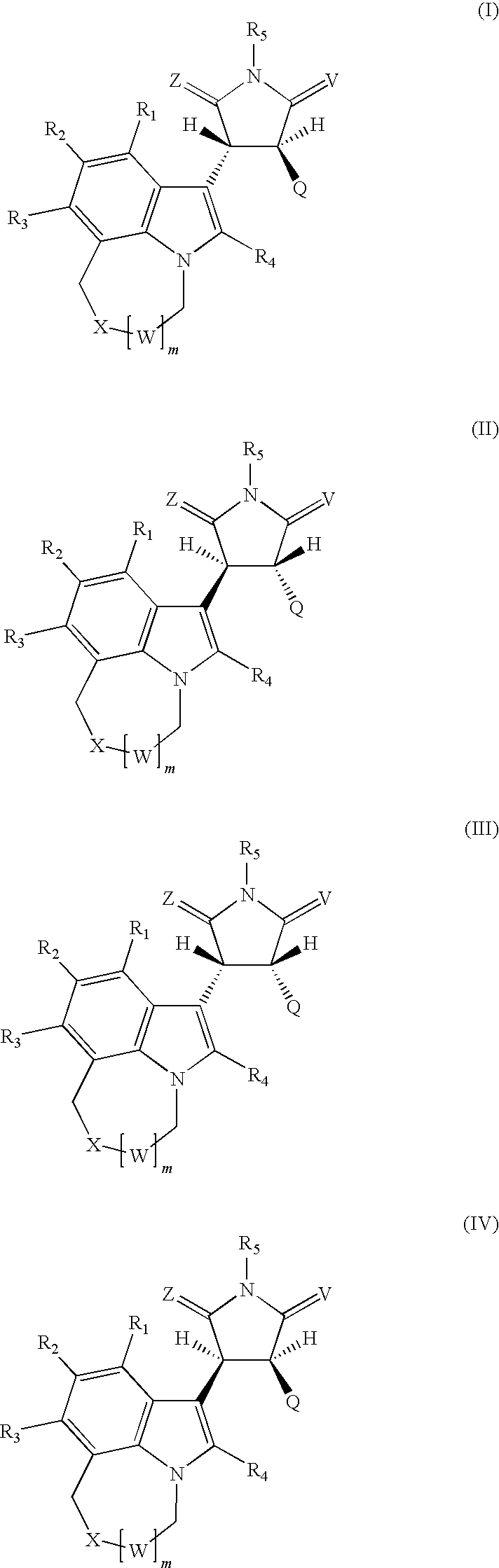
Pyrrolidinone, pyrrolidine-2,5-dione, pyrrolidine and thiosuccinimide derivatives, compositions and methods for treatment of cancer
The present invention relates to pyrrolidin-2-one, pyrrolidin-2,5-dione, pyrrolidine and thiosucciniroide compounds of formulae (I)-(IV), and methods of preparation of these compounds. The present invention also relates to pharmaceutical compositions comprising pyrrolidin-2-one, pyrrolidin-2,5-dione, pyrrolidine and thiosuccinimide compounds. The present invention provides methods of treating a cell proliferative disorder, such as a cancer, by administering to a subject in need thereof a therapeutically effective amount of a compound of pyrrolidin-2-one, pyrrolidin-2,5-dione, pyrrolidine and thiosuccinimide compound of the present invention.
Owner:ARQULE INC
1-acetoxy-3-(substituted phenyl) propen compounds preparation method
ActiveCN1738811AOrganic compound preparationCarboxylic acid esters preparationBenzeneGroup 12 element
The compounds represented by the formula (I) are produced by reacting benzene compound of the formula (IV) or (V) with alkenylidene diacetate of the formula (VI) in the presence of a catalyst comprising one or more members selected from (a) halogenated boron compounds, (b) triflate compounds of Group 11 elements, (c) halogenated compounds of Group 12 elements, and (d) triflate and halogenated compounds of tin and atomic numbers 58 and 66 to 71 elements. R<1>, R<2> = H or C1 - C10 alkyl group A = Substituted phenyl group corresponding to a compound of formula (IV) or (V), R<3>, R<4> = H or C1 - C4 alkyl group, m = 0 or 1 - 4, n = 1 to 5, k = 1 or 2.
Owner:UBE IND LTD
Carbon Black Surface-Modified with Benzene Compound and Carbon Black Dispersion Composition for Black Matrix Using the Same
InactiveUS20090182079A1Improve uniformityImprove resolutionPigmenting treatmentLayered productsOligomerTM compound
Disclosed herein are carbon black surface-modified with benzene compound of Formula 1 described in the specification and a carbon black dispersion composition for a black matrix using the carbon black. The carbon black dispersion composition uses carbon black surface-modified with the benzene compound of Formula 1, or about 0.1 to about 20% by weight of a cardo compound selected from cardo monomers, oligomers, polymers and mixtures thereof. The carbon black dispersion composition can provide improved adhesive properties, uniformity and resolution of black matrix patterns, and no undercut is formed on the black matrix patterns.
Owner:CHEIL IND INC
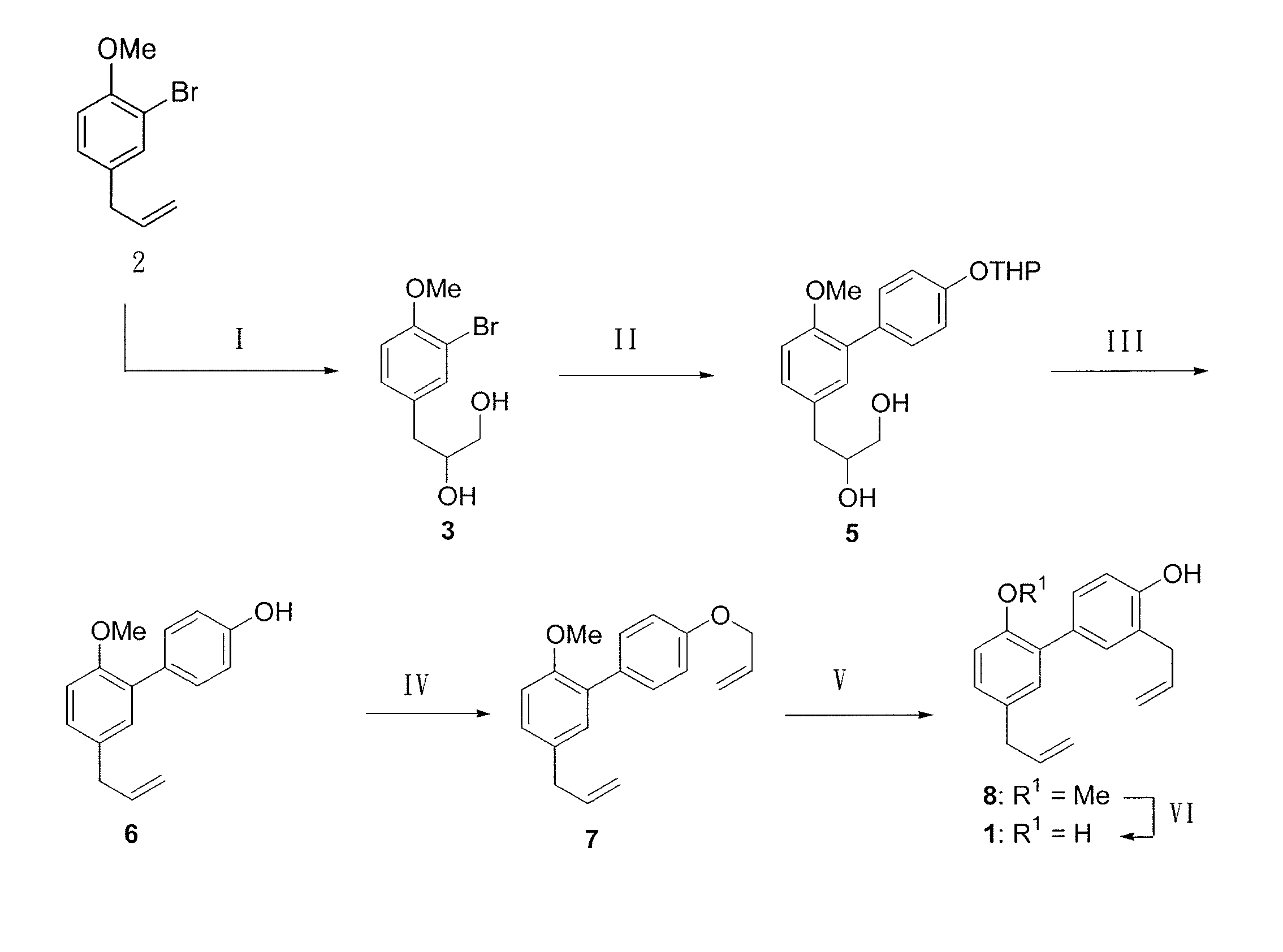
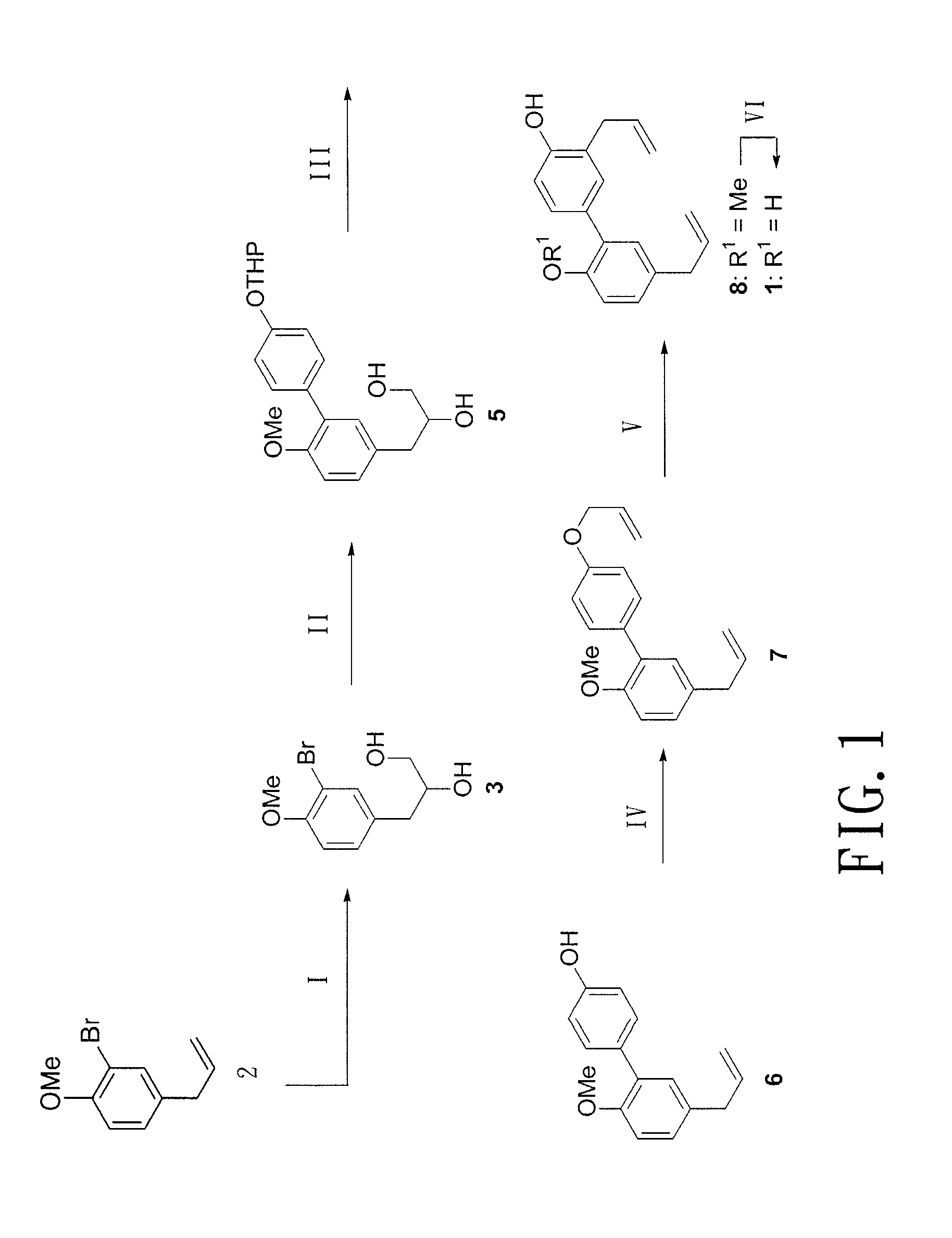
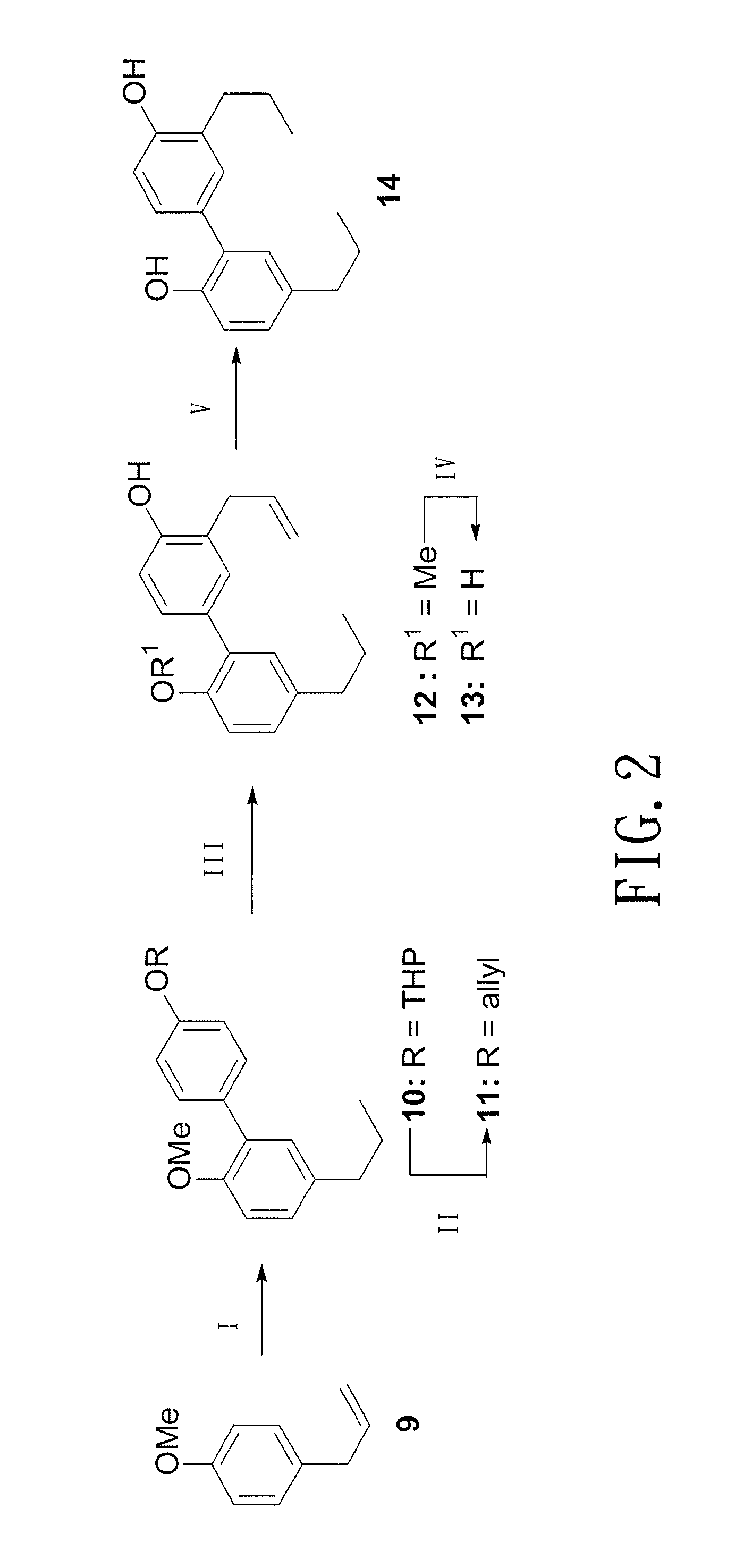
Method of producing biphenolic compound, novel biphenyl compound and synthesis method thereof, and pharmaceutical composition for treating parkinson's disease
Owner:TZU CHI UNIV
Compositions Useful for Diverting or Stopping Fluids in Subterranean Operations
PendingUS20210130678A1More sealImprove permeabilityFluid removalDrilling compositionChemical compoundPhysical chemistry
The flow of a fluid may be diverted from a high permeability zone to a low permeability zone of a subterranean formation or well sections may be temporarily isolated by use of particles comprising a mixture of (i) at least one bi-phenyl compound of Compound I, (ii) one mellitic derivative of Compound II, (iii) one chelating agent of Compound III, (iv) one polymer of Compound IV, and (v) an internal breaker for the diverting agents and other additives like gels, foams, acids, brines and various other treatment chemicals.
Owner:KAMDAR AMBRISH +1
Flexible light-transmitting metamaterial composite film and method for detecting nitrite
InactiveCN108226130ANo preprocessing requiredHigh sensitivityRaman scatteringNitrite ionComposite film
The invention discloses a flexible light-transmitting metamaterial composite film and method for detecting nitrite. A single layer of metal nanoparticle array is formed on a soft light-transmitting substrate as a metamaterial layer, and a p-aminothiophenol self-assembled monomolecular film covers the single layer of metal nanoparticle array as a nitrite sensitive film; under the excitation of visible / near infrared light, surface plasmon resonance is generated on the metamaterial layer; p-aminothiophenol in the nitrite sensitive film is induced to be converted into an azobenzene compound underthe synergistic action of surface ions of the metamaterial layer and sub-salt nitrate ions; since the molecules of the azobenzene compound and the molecules of the p-aminothiophenol have a significantdifference in the aspect of Raman spectrum, the reaction is directly monitored by using the Raman spectrum, and the specific recognition and detection of nitrite ions are indirectly realized. The method utilizes the special optical properties possessed by the metamaterial and combines the surface selectively of sub-salt nitric acid to catalyze aniline so as to generate the azobenzene, thereby realizing the rapid detection of nitrite.
Owner:ZHEJIANG UNIV
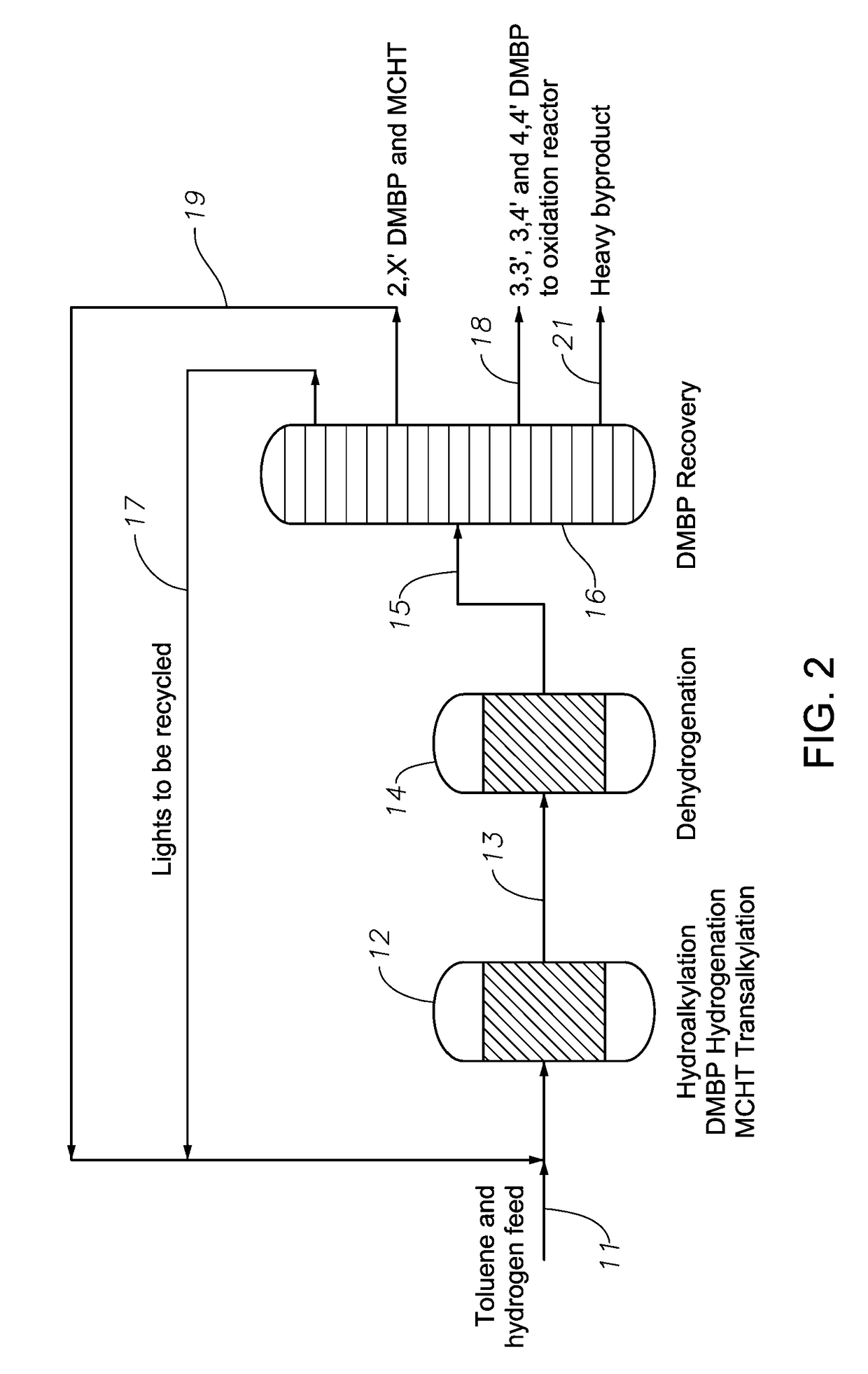
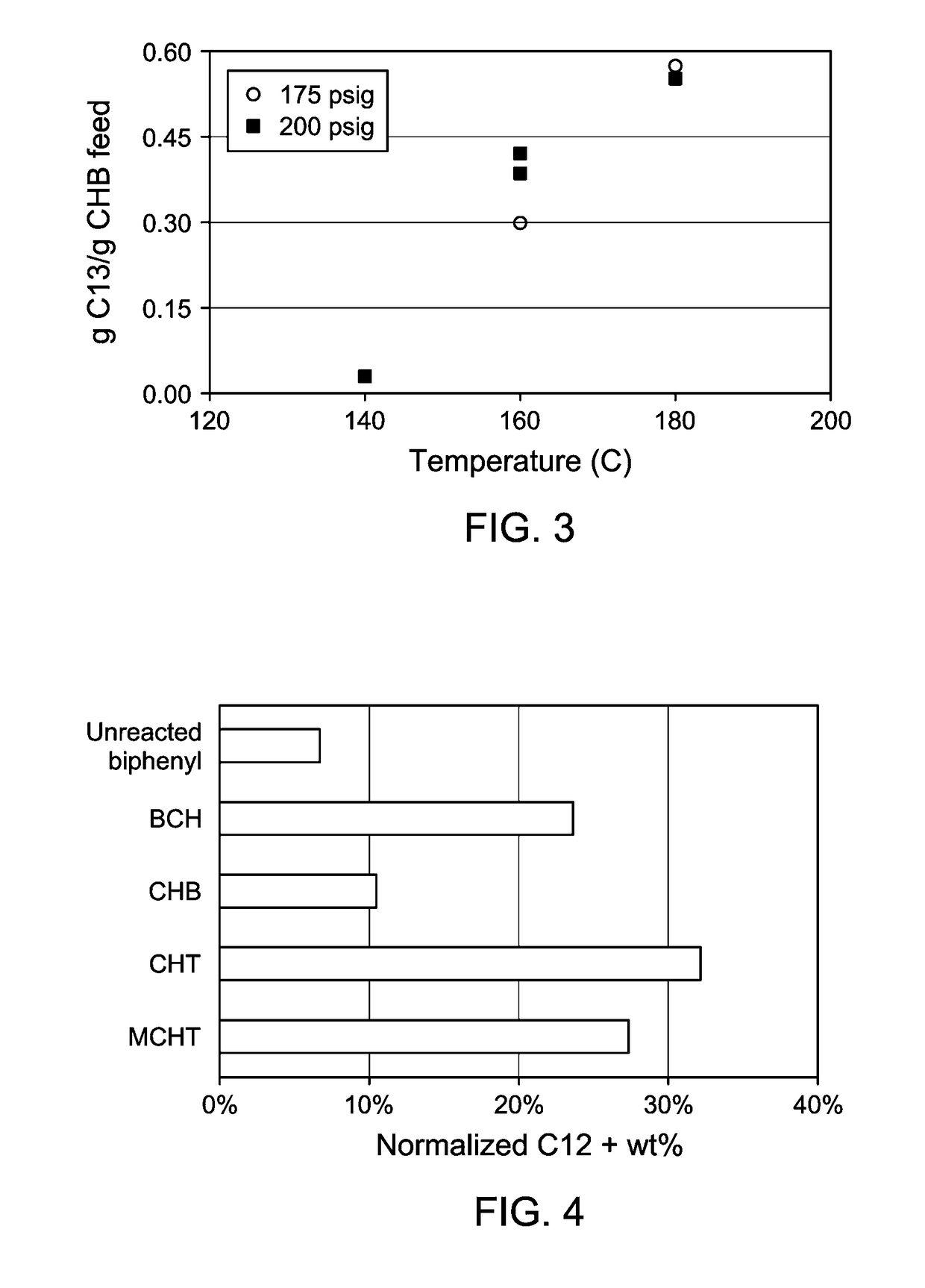
Production of biphenyl compounds
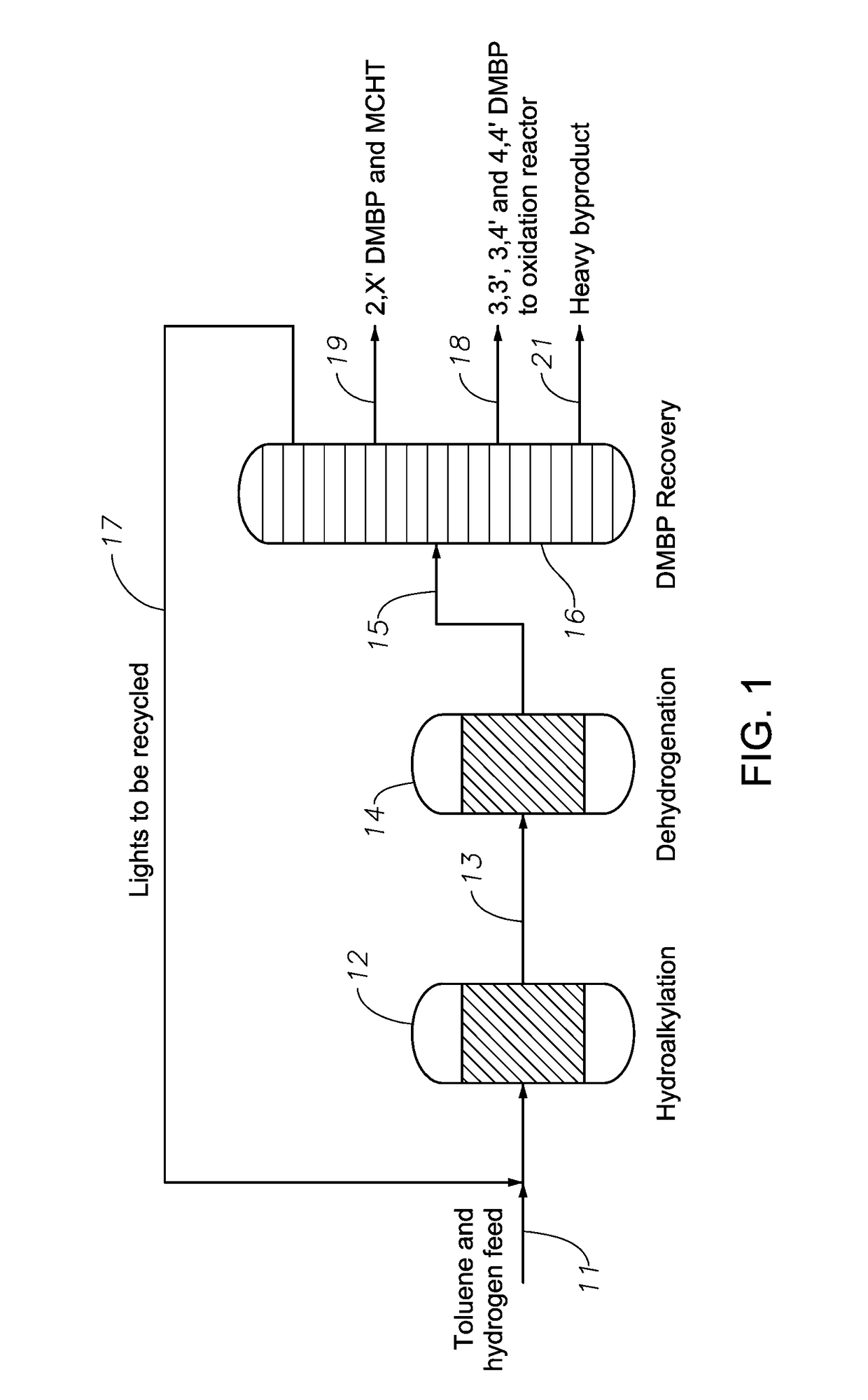
ActiveUS9708230B2Lower conversion rateMolecular sieve catalystOrganic compound preparationHydrocotyle bowlesioidesDehydrogenation
In a process for producing biphenyl compounds, a Cn aromatic hydrocarbon may be hydroalkylated to give C2n cycloalkylaromatic compounds and byproduct Cn saturated cyclic hydrocarbons. The C2n cycloalkylaromatic compounds are dehydrogenated to provide the biphenyl compounds. The Cn saturated cyclic hydrocarbons may also be dehydrogenated back to the corresponding Cn aromatic hydrocarbon, which may be recycled to provide additional feed. Although both the intermediate C2n cycloalkylaromatic compounds and the byproduct Cn saturated cyclic hydrocarbons should be dehydrogenated, at least part of the dehydrogenation of the Cn saturated cyclic hydrocarbons should take place in the absence of C2n or greater hydrocarbons. Thus, dehydrogenation of the byproduct Cn saturated cyclic hydrocarbons should take place at least in part separately from dehydrogenation of the C2n cycloalkylaromatic compounds.
Owner:EXXONMOBIL CHEM PAT INC
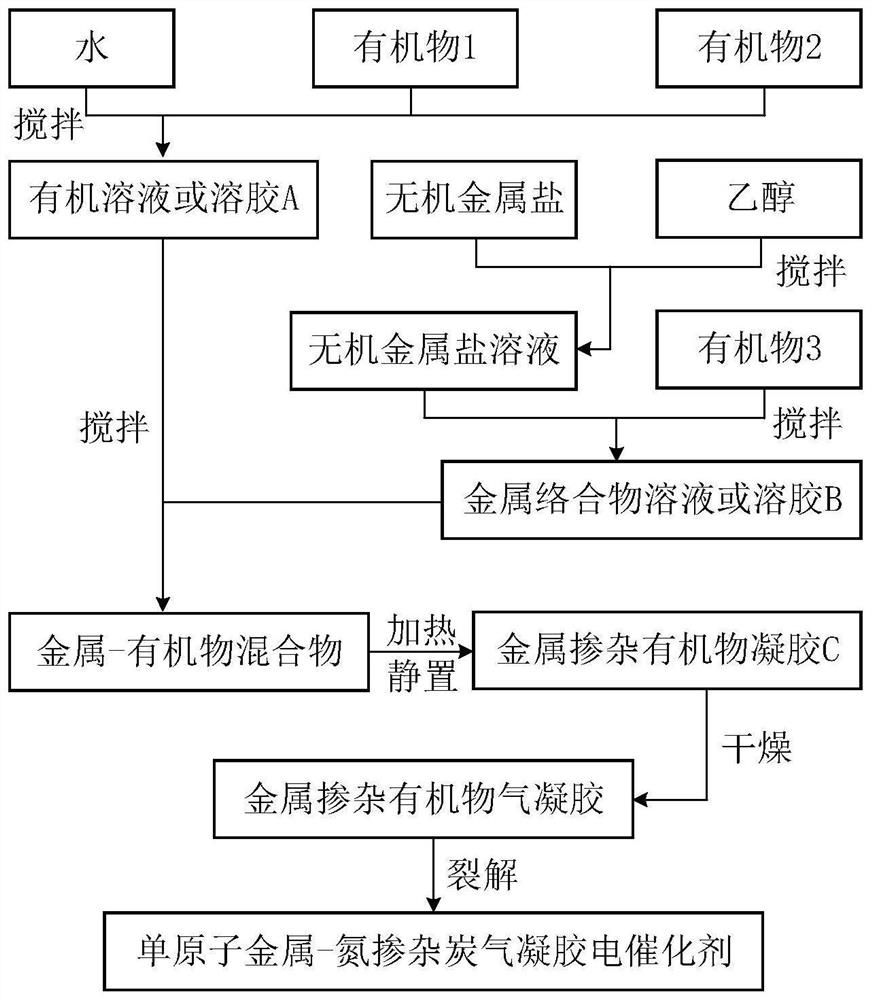
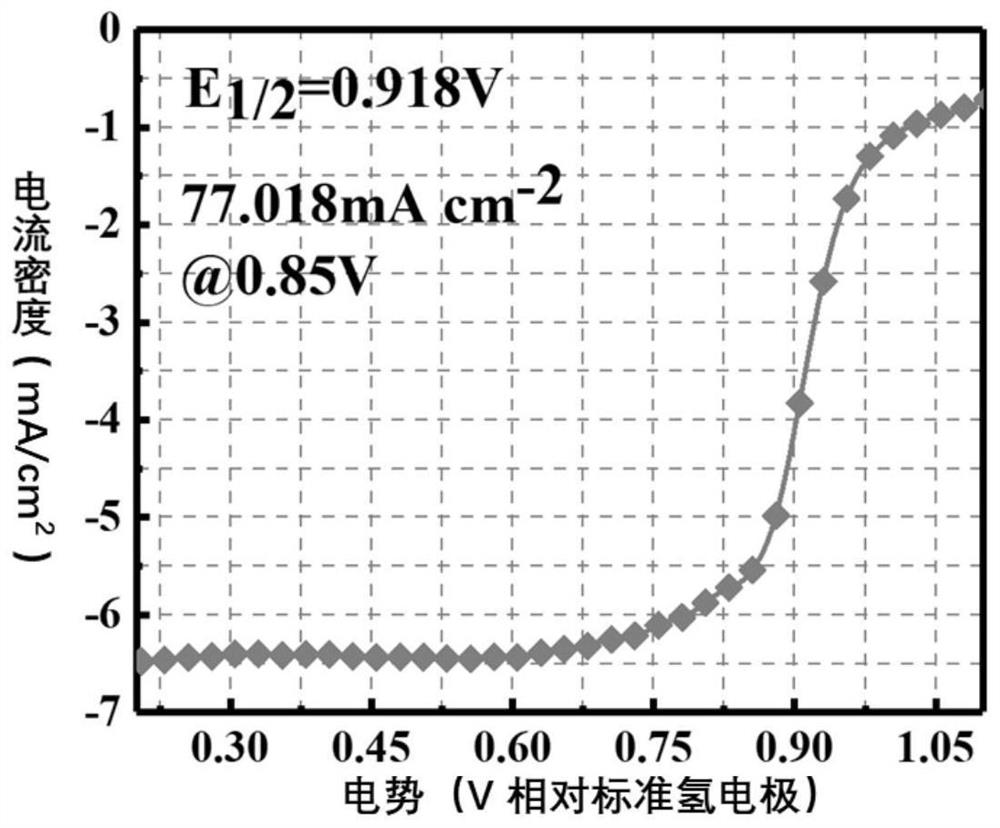
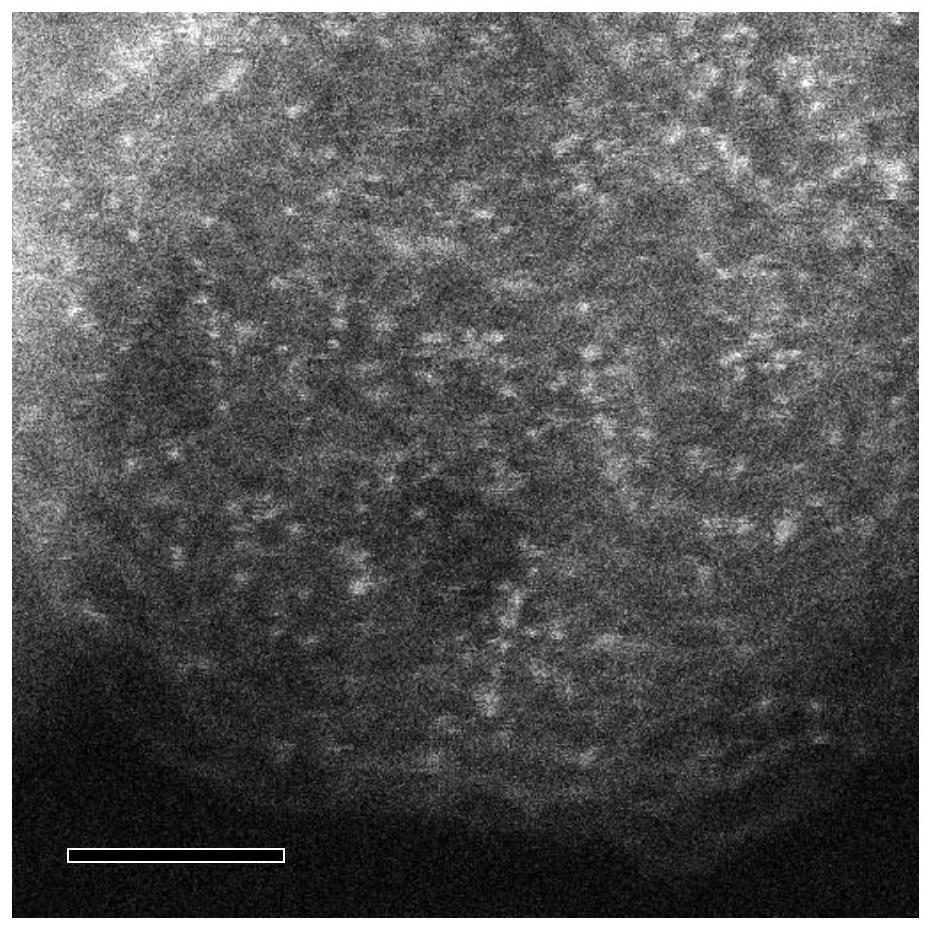
Preparation method of monatomic metal-nitrogen doped carbon aerogel electrocatalyst
ActiveCN113584514AEasy to prepareLarge specific surface areaCell electrodesElectrolytic organic productionPtru catalystHigh activity
The invention discloses a preparation method of a monatomic metal-nitrogen doped carbon aerogel electrocatalyst, and aims to meet the urgent demand of the field of electrocatalysis on large-scale preparation of a low-cost and high-activity monatomic electrocatalyst. According to the technical scheme, the preparation method comprises the following steps: firstly, preparing an organic solution or sol A from aldehyde organic matters containing aldehyde groups, polyhydroxy benzene compounds or polyamino benzene compounds; preparing a metal complex solution or sol B from an inorganic metal salt and an organic matter which can generate a complex with the inorganic metal salt or can catalyze metal salt ions to form metal cation sol; mixing and heating A and B to obtain metal-doped organic matter gel C; removing liquid in the C to obtain metal-doped organic matter aerogel; and cracking the metal doped organic matter aerogel to obtain the monatomic metal-nitrogen doped carbon aerogel electrocatalyst. The acid pickling step is avoided, active sites are generated in one step in situ in the cracking process, the method is simple and low in cost, and the electrocatalyst prepared through the method is very high in catalytic activity.
Owner:NAT UNIV OF DEFENSE TECH
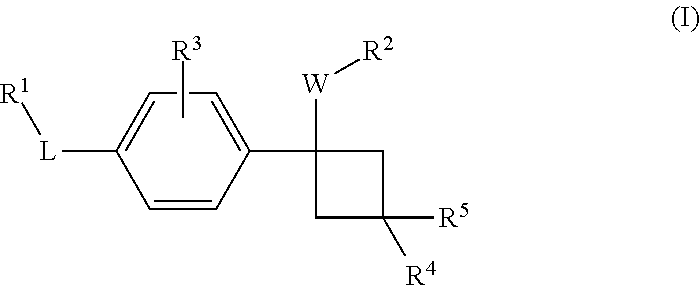
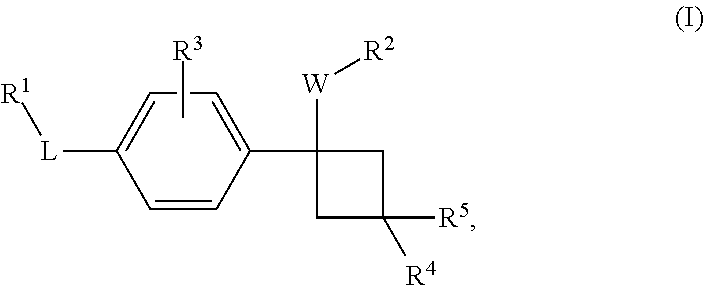
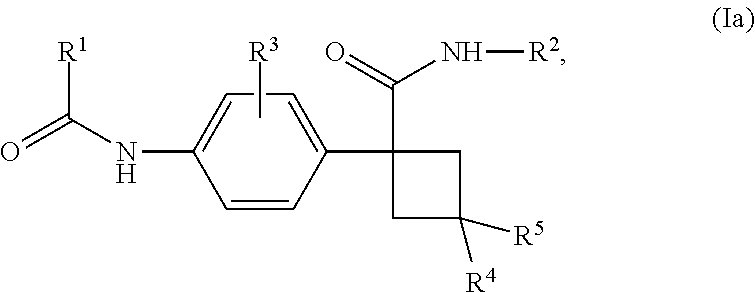
Novel substituted cyclobutylbenzene compounds as indoleamine 2,3-dioxygenase (IDO) inhibitors
Disclosed herein is a compound of formula (I), or a pharmaceutically acceptable salt thereof: (Formula (I)). Also disclosed herein are uses of a compound disclosed herein in the potential treatment or prevention of an IDO-associated disease or disorder. Also disclosed herein are compositions comprising a compound disclosed herein. Further disclosed herein are uses of a composition in the potential treatment or prevention of an IDO-associated disease or disorder.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com