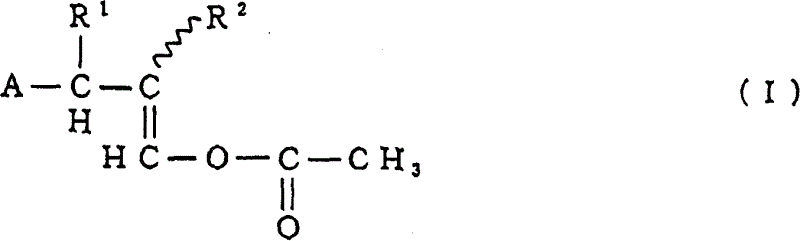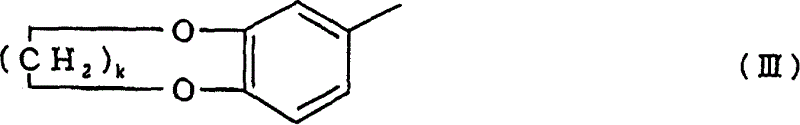1-acetoxy-3-(substituted phenyl) propen compounds preparation method
A technology for acetoxy and benzene compounds, applied in the field of preparation of 1-acetoxy-3-propene compounds, which can solve the problems of low and unsatisfactory yields of target compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The 1-acetoxy-3-(substituted phenyl)propene compound prepared according to the preparation method of the present invention is based on the asymmetric carbon atom and / or double bond contained in the compound represented by the above general formula (I), of course Contains various stereoisomers.
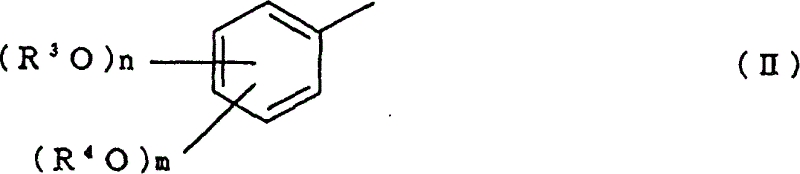
[0047] In order to prepare the 1-acetoxy-3-(substituted phenyl) propene compound of the present invention, the method of the present invention comprises: in the presence of the specific catalyst described in detail later, making the compound obtained from the above general formula (IV) and (V) One selected from the group of benzene compounds shown is reacted with the alkenylene diacetate represented by the above general formula (VI). The benzene compound of formula (IV) and (V) corresponds to the substituted phenyl represented by general formula (II) and (III), and the alkenylene diacetate represented by general formula (VI) is in general formula (I) In combination with the A b...
Embodiment 1
[0085] In an argon atmosphere, 1,2-methylenedioxybenzene (6.83 g, 56.0 mmol) and 3,3-diacetoxy- A mixed solution of 2-methylpropene (1.05 g, 5.6 mmol) was mixed with boron trifluoride diethyl ether complex (74 mg, 0.52 mmol). The mixture was stirred at 23° C. for 1 hour, ethyl acetate (50 ml) was mixed into the resulting reaction solution, the organic layer formed in the reaction solution was separated, washed three times with water (50 ml), and dried over anhydrous sodium sulfate , and the solvent was distilled off. The residue was subjected to silica gel column chromatography, and 1-acetoxy-2-methyl-3-(3,4-methylenedioxy 1.15 g of phenyl)propene was collected as white crystals. The isolated yield of the obtained target compound was 88%.
[0086] The physical property values of 1-acetoxy-2-methyl-3-(3,4-methylenedioxyphenyl)propene are expressed as follows:
[0087] 1 H-NMR (300MHz, CDCl 3 ) δ = 1.56 (3H, d, J = 1.5Hz), 2.15 (3H, s), 3.18 (2H, s), 5.92 (2H, s), 6.63 (...
Embodiment 2
[0094] In an argon atmosphere, 1,2-methylenedioxybenzene (6.83 g, 55.97 mmol) and 3,3-diacetoxy- A mixed solution of 2-methylpropene (0.96 g, 4.88 mmol) was mixed with a boron trifluoride diethyl ether complex (77 mg, 0.54 mmol). The mixed solution was stirred at 23° C. for 1 hour, acetonitrile (100 ml) was mixed with the obtained reaction solution, the mixture was subjected to high performance liquid chromatography, and the reaction solution was analyzed by the absolute calibration curve method. As a result, the yield of 1-acetoxy-2-methyl-3-(3,4-methylenedioxyphenyl)propene was 97.1%. In addition, 5.86 g of unreacted 1,2-methylenedioxybenzene was contained in the reaction liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com