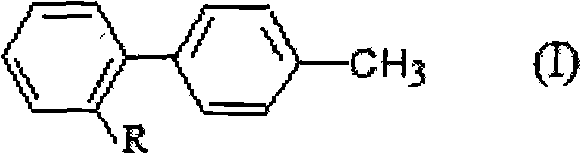
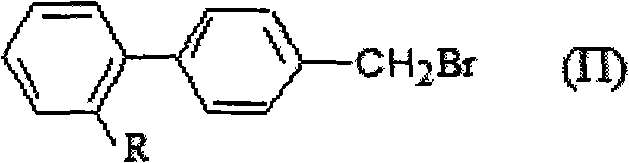
Method for preparing and recovering aromatic methyl diphenyl compound
A technology for aromatic methyl biphenyl and aromatic bromomethyl biphenyl, which is applied in the field of industrial preparation and recovery of aromatic methyl biphenyl compounds, can solve problems such as high price and limitation, and achieve the effect of improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0034] Add 50g (0.2588mol) of 4'-methyl-2-cyanobiphenyl (OTBN) and 300ml of dichloromethane into a four-neck flask, set the internal temperature at 35-40°C, add light, and add it dropwise within 3 hours 22.8g (0.1425mol) bromine, 30g (content 27.5%, 0.2426mol) hydrogen peroxide, and then keep warm at 35-40°C for 3 hours, take a sample, and analyze by HPLC. The mixture was found to contain 5.1% 4'-methyl-2-cyanobiphenyl, 85.5% 4'-bromomethyl-2-cyanobiphenyl, 9.2% 4'-dibromomethyl-2-cyanobiphenyl benzene.
[0035] Then, the water layer was separated, and the organic phase was distilled out of dichloromethane, and 250ml of toluene was added for separation and purification to obtain 4'-bromomethyl-2-cyanobiphenyl with a purity of 53.8g (HPLC) of 97.8%. The yield was 76.4%. % (based on 4'-methyl-2-cyanobiphenyl). Then, the toluene in the mother liquor after separation of the product was distilled off to obtain 12.2 g of the mixture. HPLC analysis found that the mixture contained...
Embodiment 1
[0037] Add 5g (0.0769mol) of zinc powder, 6g (0.1121mol) of ammonium chloride, and 100ml of toluene into a three-necked flask, set the internal temperature at 45-50°C, and stir; in addition, dissolve the mother liquor mixture obtained in the above preparation example in 60ml of toluene , and then drop it into a three-necked flask for about 1 hour, then keep warm at 45-50°C for 18 hours, filter, wash, remove impurities such as inorganic salts, distill toluene, and then fractionally distill to obtain 6.7g of 4'-methyl-2- Cyanobiphenyl, GC analysis purity 97.5%.
Embodiment 2
[0039] According to the method of Preparation Example 1, 14.1g of the mixture was obtained. According to HPLC analysis, the mixture contained 30.1% 4'-methyl-2-cyanobiphenyl, 29.7% 4'-bromomethyl-2-cyanobiphenyl, 39.7% % 4'-Dibromomethyl-2-cyanobiphenyl.
[0040]Add 8g (0.1231mol) of zinc powder, 12g (0.0909mol) of ammonium sulfate, and 200ml of toluene into a three-necked flask, set the internal temperature at 70-75°C, and stir; in addition, dissolve the above mother liquor mixture in 80ml of toluene, and then dissolve it for about 1 hour. Add it dropwise to a three-necked flask, keep warm at 70-75°C for 12 hours, filter, wash, remove impurities such as inorganic salts, distill toluene, and then fractionally distill to obtain 8.1g of 4'-methyl-2-cyanobiphenyl, GC analysis purity 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com