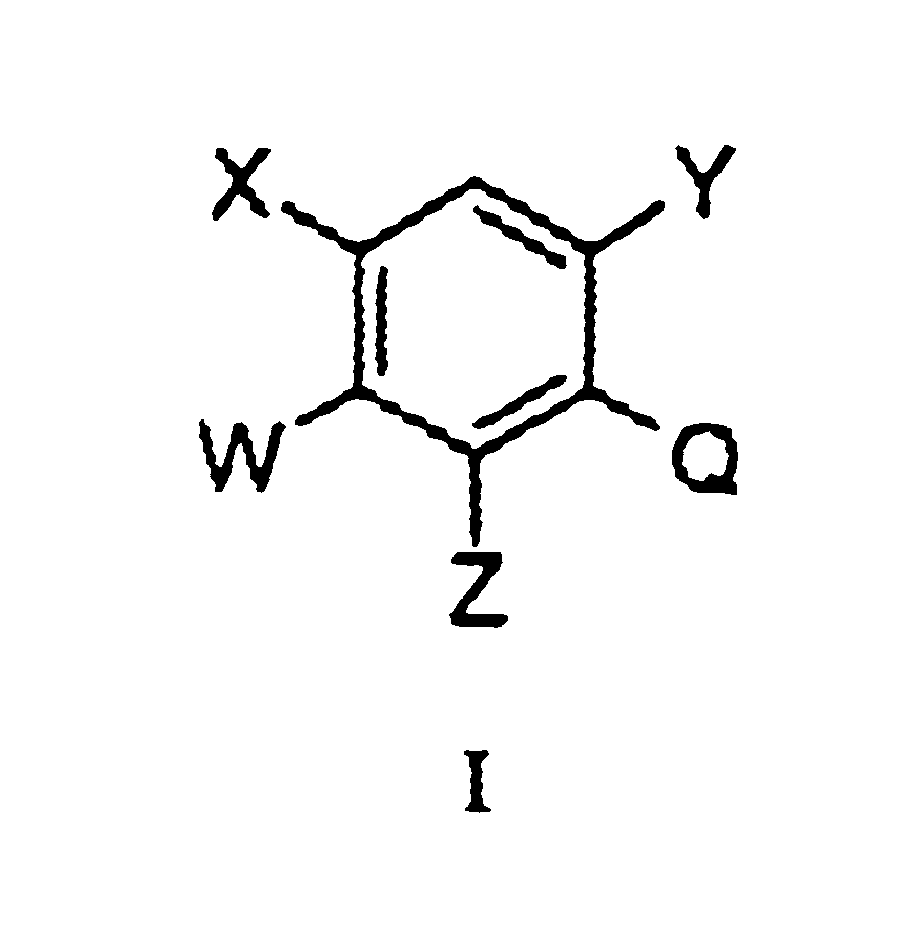
Substituted benzene compounds, process for their preparation and herbicidal and defoliant composition containing them
A compound, haloalkyl technology, applied in the field of herbicide and defoliant composition, can solve the problem of not describing the general structure of the compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of 3-(4-chloro-6-fluoro-3-methoxy-2-nitrophenyl)-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Compound No. 1 -1)
[0063] 3-(4-Amino-6-fluoro-3-methoxyphenyl)-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (10 .0g, 29.5mmol) was slowly added to a stirred mixture of concentrated sulfuric acid (36ml) and concentrated nitric acid (4ml). The solution was then slowly warmed to room temperature and allowed to stir for 2 hours. This solution was added to ice water to give a pale yellow precipitate which was isolated by filtration to give the title compound (9.1 g). The NMR data for this compound are listed in Table XVIII. Example 2
Embodiment 2
[0064] 3-(4-Chloro-6-fluoro-3-methoxy-2-nitrophenyl)-1-methyl-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione Preparation (Compound No. 1-5)
[0065] 3-(4-Chloro-6-fluoro-3-methoxy-2-nitrophenyl)-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (9g, 23. 5mmol) was dissolved in dimethylformamide (90ml), and to this solution were added potassium carbonate (3.9g, 28.2mmol) and dimethyl sulfate (10.2g, 47mmol) with stirring. The solution was stirred at room temperature for 12 hours and water was added. The product was extracted into ethyl acetate and the organic layer was washed with water and dried over anhydrous sodium sulfate. Removal of the solvent gave the crude product, which was purified by silica gel column chromatography. The column was eluted with dichloromethane to give the title compound (7.8g).
Embodiment 3
[0067] Preparation of 3-(2-amino-4-chloro-6-fluoro-3-methoxyphenyl)-1-methyl-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (Compound No. 1-4)
[0068] 3-(4-Chloro-6-fluoro-3-methoxy-2-nitrophenyl)-1-methyl-6-trifluoromethyl-2,4(1H,3H)-pyrimidinedione (7.5g, 18.9mmol) was dissolved in acetic acid (75ml), and 4.2g (75.6mmol) of iron powder was added. The solution was stirred at room temperature under a nitrogen atmosphere for 6 hours and water was added. Extract with ethyl acetate. The organic layer was washed with water, brine, and dried over anhydrous sodium sulfate, followed by evaporation to give the title compound (6.8 g).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com