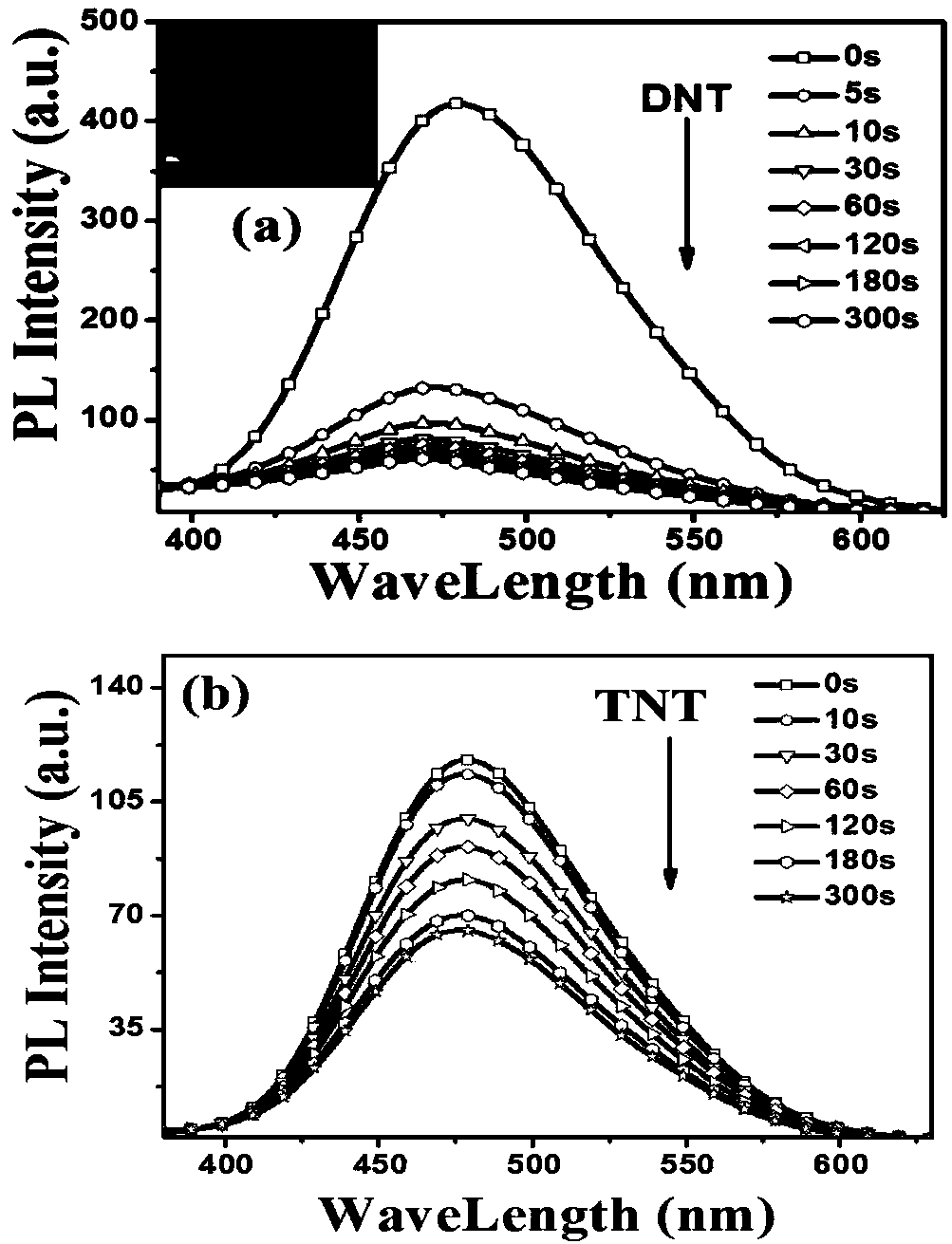
Aggregation-induced luminescent organic fluorescent small-molecule material and application thereof in DNT and TNT gas fluorescence detection
An aggregation-induced luminescence, small molecule technology, applied in the field of fluorescence sensing, can solve the problems of low fluorescence efficiency and poor photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of compound BTT
[0041]
[0042] Synthesis of 4-N,N-diphenylamine-3,5-bis-(4-bromophenyl)-4H-1,2,4-triazole
[0043] Under the protection of argon, the monomer aminotriphenylamine (0.54g, 2mmol), 1,2-bis((4-bromophenyl)chloromethylene)hydrazine (0.87g , 2mmol) and 15mL of N,N-dimethylaniline, reacted at 135°C for 12h, then added 2M HCl (30mL) solution. After reacting again for 30 minutes, a large amount of precipitates were generated. After vacuum filtration, washing and drying, the crude reaction product was purified and separated by column chromatography with a mixed solvent of dichloromethane:ethyl acetate / 5:1 (volume ratio) as a developer. , a white solid 5 was obtained with a reaction yield of 67%.
[0044] 1 H NMR (500MHz, CDCl 3 ): δ8.20(d, 1H), 7.75(d, 1H), 7.57(d, 2H), 7.51(t, 1H), 7.43(d, 4H), 7.38(t, 1H). MALDI-TOF MS(mass m / z):620.00[M] + .
[0045] Synthesis of BTT
[0046] 622.45mg 4-N,N-diphenylamine-3,5-bis-(4-brom...
Embodiment 2
[0048] Embodiment 2: the synthesis of compound BPT:
[0049]
[0050] 9-9H-carbazole-3,5-bis-(4-bromophenyl)-4H-1,2,4-triazole
[0051] Under argon protection, the monomer 4-(9H-carbazole)aniline (0.52g, 2mmol), 1,2-bis((4-bromophenyl)chloromethylene ) hydrazine (0.87g, 2mmol) and 15mL N,N-dimethylaniline were reacted at 135°C for 12h, and then 2M HCl (30mL) solution was added. After reacting again for 30 minutes, a large amount of precipitates were generated. After vacuum filtration, washing and drying, the crude reaction product was purified and separated by column chromatography with a mixed solvent of dichloromethane:ethyl acetate / 5:1 (volume ratio) as a developer. , a white solid 5 was obtained with a reaction yield of 67%.
[0052] 1 H NMR (500MHz, CDCl 3 ; 25°C, TMS) δ=8.20(d, J=7.7Hz, 2H; Ar H), 7.75(d, J=8.5Hz, 2H; Ar H), 7.57(d, J=8.5Hz, 4H; Ar H), 7.51(t, J=7.7Hz, 2H; Ar H), 7.43(d, J=8.4Hz, 8H; Ar H), 7.38(t, J=7.4Hz, 2H; Ar H).MALDI- TOFMS(mass m / z):620.0...
Embodiment 3
[0056] Embodiment 3: the fluorescence emission spectrum of the BTT solution of normalized different water and tetrahydrofuran volume ratio
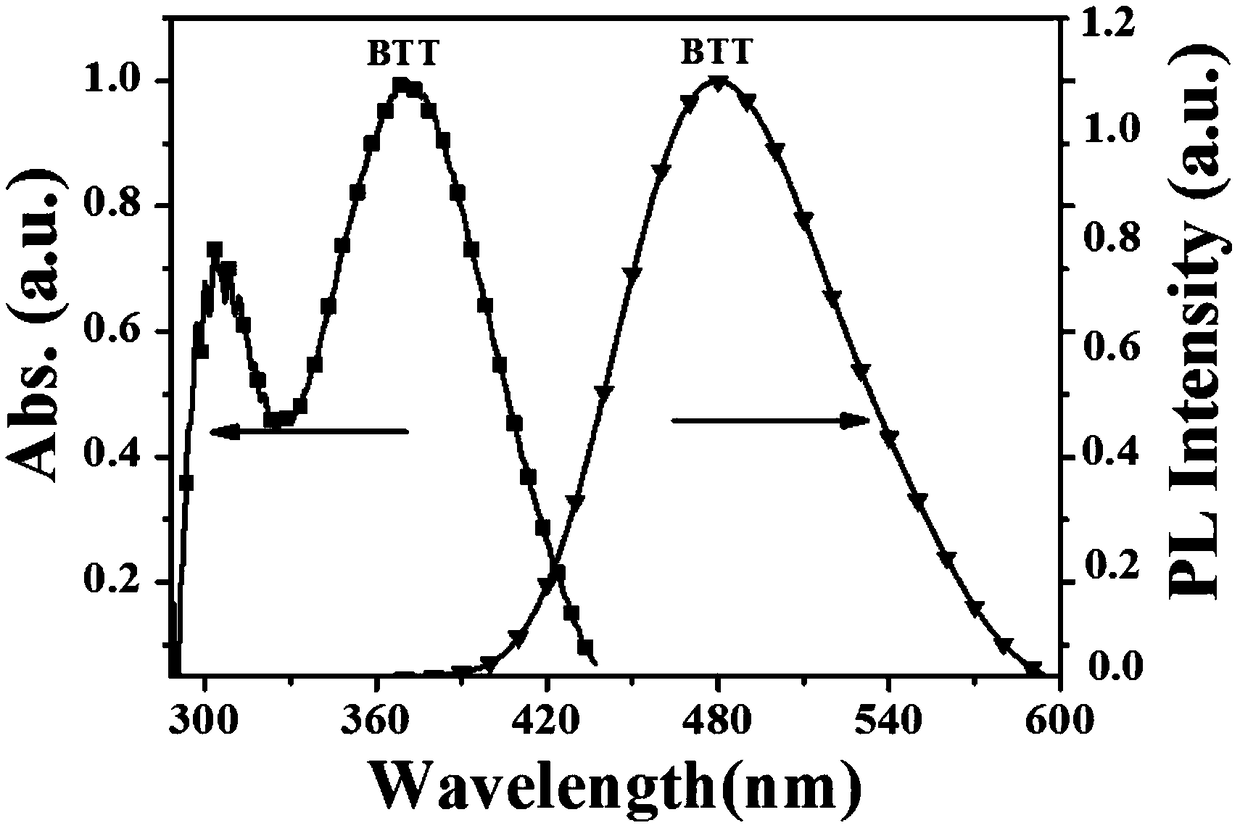
[0057] Prepare ten 50mL volumetric flasks, add 0.5mg BTT to them respectively, and then add water and tetrahydrofuran mixed solvent with the volume percentage of water increasing from 0 to 90% to 50mL. Take 3mL and add it to a quartz cuvette, and use a fluorescence spectrometer to record the fluorescence emission spectrum of the solution when the volume percentage of the configured water increases from 0 to 90%. We can see from the figure that as the proportion of water increases, the fluorescence intensity increases and the brightness increases. This phenomenon also demonstrates the AIE property of our material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com