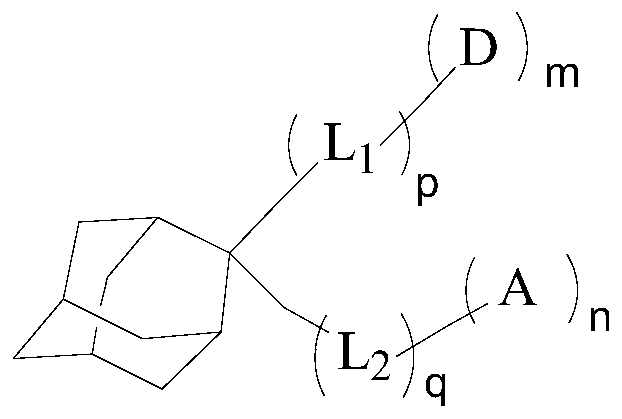
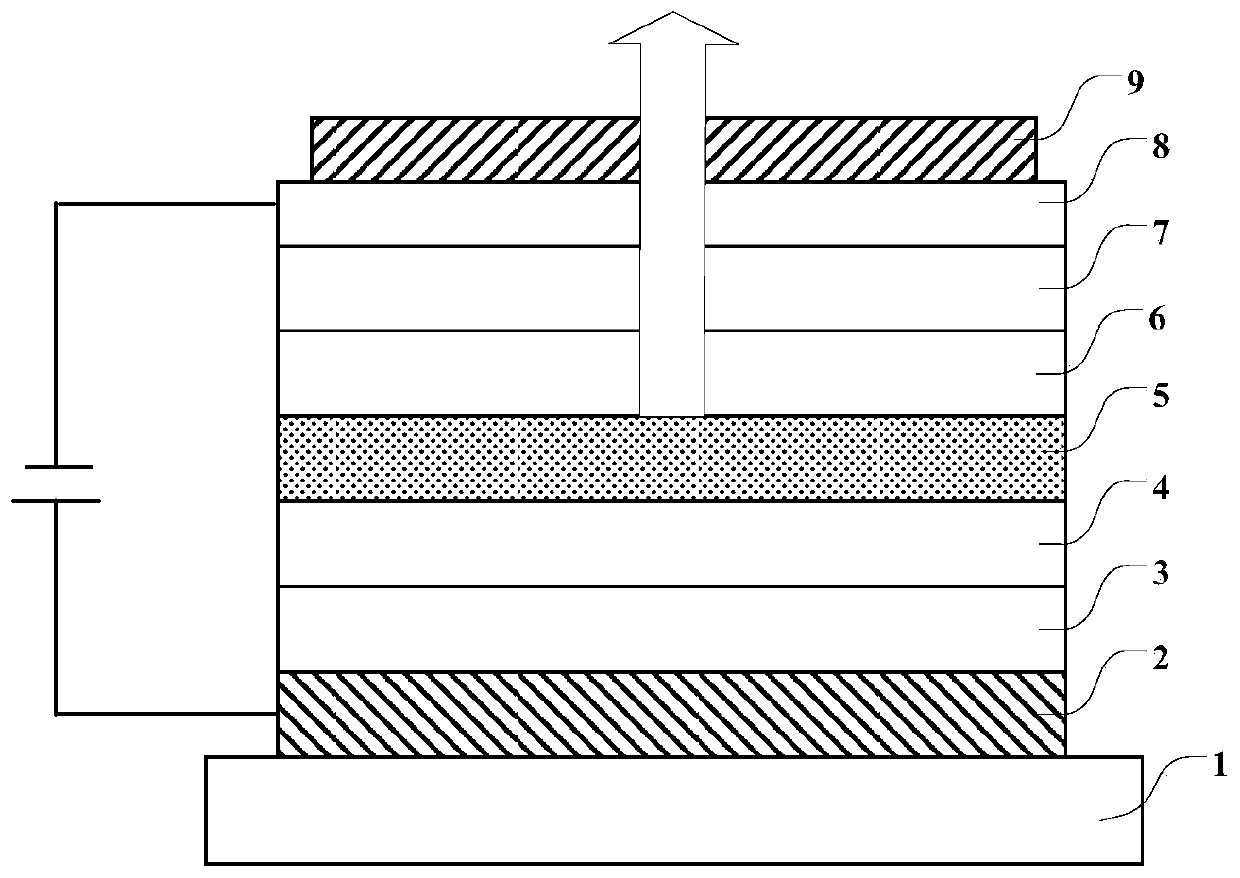
Compound, display panel and display device
A display panel and compound technology, which is applied in the fields of compounds, display panels and display devices, can solve the problem of difficulty in developing doping materials, etc., and achieve the effects of improving luminous efficiency, broadening exciton recombination area, and improving equilibrium migration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Synthesis of Compound H002
[0086]
[0087] In a 250ml round bottom flask, the intermediate product H002-1 (15mmol) and potassium acetate (40mmol) were mixed with dry 1,4-dioxane (60ml), Pd(PPh 3 ) 2 Cl 2 (0.4mmol) and pinacol diboronate (25mmol) were mixed, and stirred at 90° C. under a nitrogen atmosphere for 48 hours (h). The resulting intermediate was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography. The product yielded intermediate H002-2.
[0088] In a 250ml round bottom flask, H002-2 (10mmol), 3-bromo-9-phenyl-9H-carbazole (12mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added to a mixture of toluene (30ml) / ethanol (20ml) and potassium carbonate (12mmol) aqueous solution (10ml), and the reaction was refluxed under nitroge...
Embodiment 2
[0092] Synthesis of Compound H018
[0093]
[0094]In a 250ml round bottom flask, 2-adamantanone (15mmol), 5-aminopyrimidine (40mmol), HCl (20ml) were refluxed for 14 hours under an argon atmosphere to obtain the bisaminophenyladamantane product H018-1 .
[0095] In a 250ml round bottom flask, the intermediate product H018-1 (15mmol) and NaNO 2 (40mmol), HCl (20ml), stirred at 0°C for 6 hours under argon atmosphere. Further KI (40 mmol) was added, and stirred at 0° C. under argon atmosphere for 48 hours. The resulting intermediate was added to water and filtered through a pad of celite, the filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography to obtain intermediate Product H018-2.
[0096] In a 250ml round bottom flask, the intermediate product H018-2 (15mmol) and potassium acetate (80mmol) were mixed with dry 1,4-di...
Embodiment 3
[0099] Synthesis of Intermediate H075-1
[0100]
[0101] In a 250ml round bottom flask, 2-adamantanone (15mmol), aniline (40mmol), and HCl (20ml) were refluxed for 14 hours under an argon atmosphere to obtain the bisaminophenyladamantane product H075-0.
[0102] In a 250ml round bottom flask, the intermediate product H075-0 (15mmol) and NaNO 2 (40mmol), HCl (20ml), stirred at 0°C for 6 hours under argon atmosphere. Further KI (40 mmol) was added, and stirred at 0° C. under argon atmosphere for 48 hours. The resulting intermediate was added to water and filtered through a pad of celite, the filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude product was purified by silica gel column chromatography to obtain intermediate Product H075-1.
[0103] Synthesis of Compound H075
[0104]
[0105] In a 250ml round bottom flask, 2,2-bis(4-iodo-phenyl)-adamantane (15mmol), cuprou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com