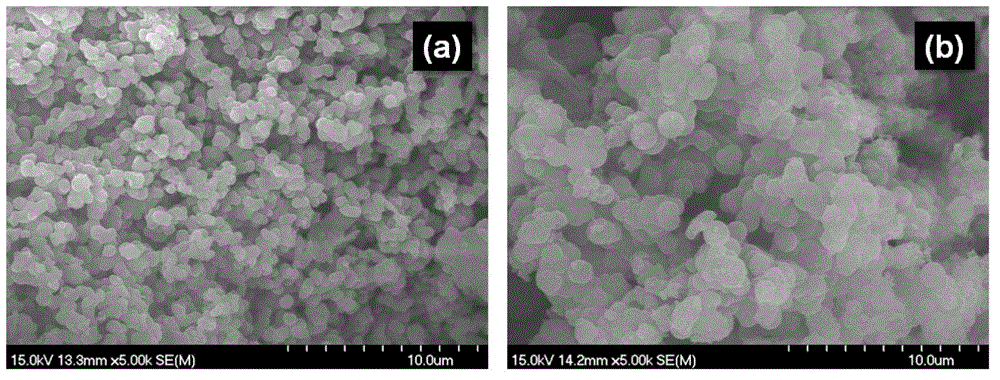
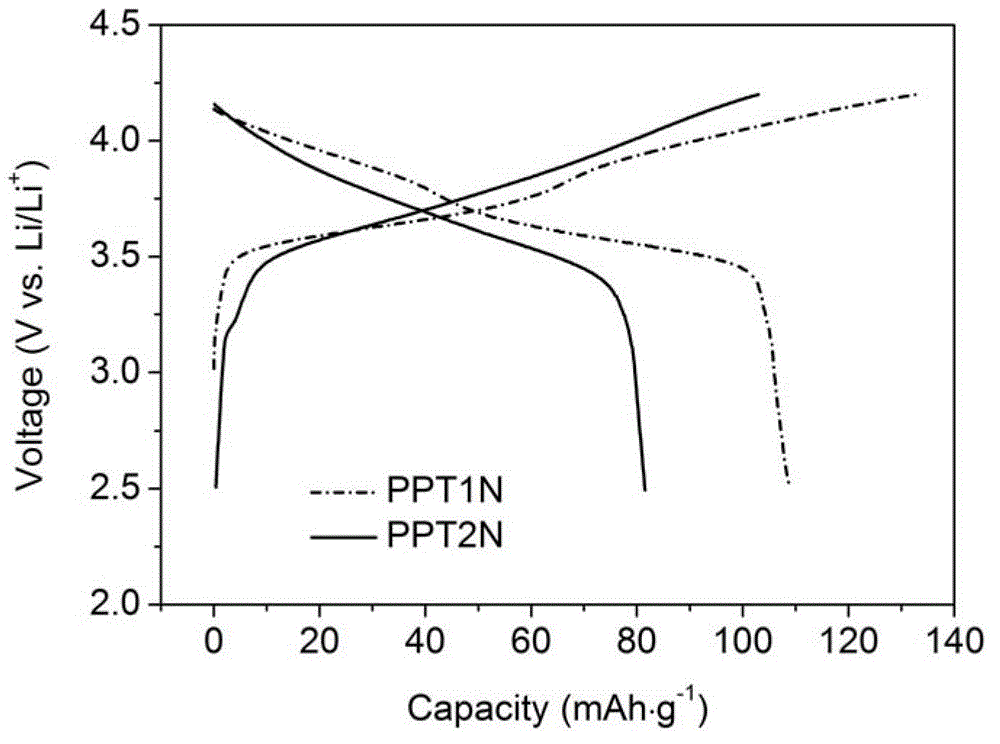
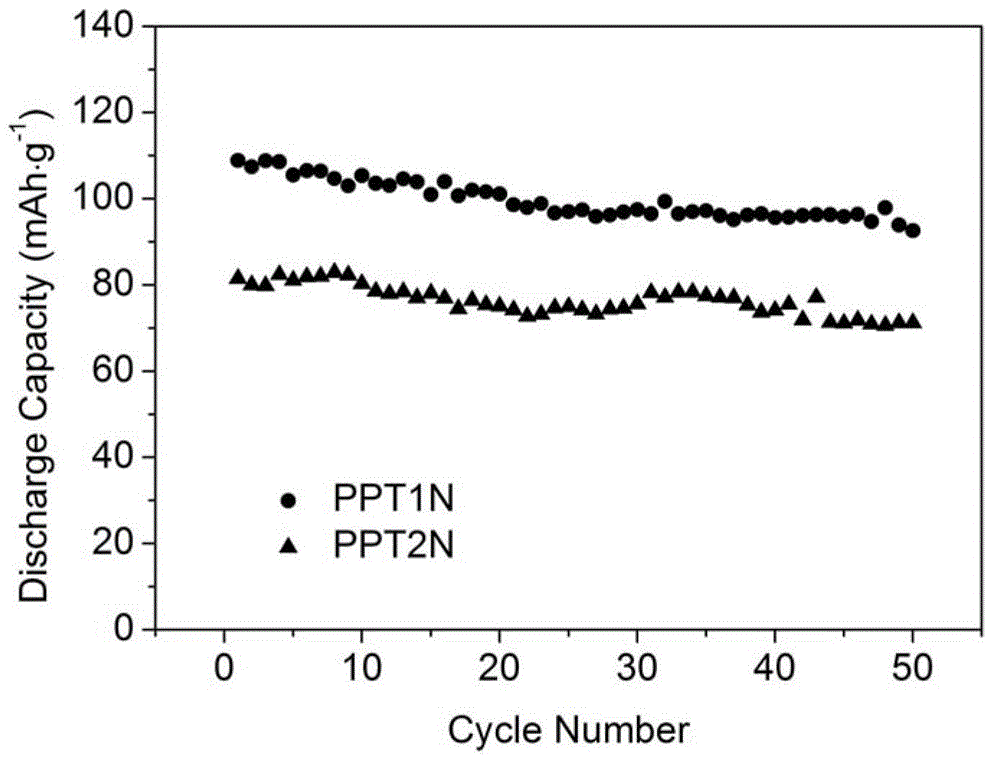
Application of triphenylamine derivative polymer as lithium ion batteries cathode material
A lithium-ion battery, cathode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as large particles and inter-particle agglomeration, and achieve high charge-discharge specific capacity, significant charge-discharge voltage platform, good fast charge and discharge. The effect of discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] PPT1N
[0035] PT1N monomer synthesis. Add 30 mL of anhydrous tetrahydrofuran to a previously dried 250 mL three-neck flask, and then add excess magnesium powder (3.02 g). 2-Bromothiophene (6 mL) was added dropwise while stirring, and the reaction released a lot of heat. To prevent the temperature from being too high, the temperature should be cooled with a cold water bath to control the reaction to proceed steadily for 5 hours. The prepared Grignard reagent suspension was quickly transferred to a dry constant pressure separatory funnel, and added dropwise to 50 mL of 4,4',4"-tribromotriphenylamine (5 g) and 1,1'- In the dry tetrahydrofuran solution of [bis(diphenylphosphino)ferrocene]palladium dichloride (0.5g) catalyst, the temperature of the reaction solution is controlled to be 60°C, and stirred for 12h under a nitrogen atmosphere to reach the end of the reaction. The reaction is performed with saturated chlorine The ammonium chloride aqueous solution is terminate...
Embodiment 2
[0045] PPT2N
[0046] PT1N tribromide synthesis. 30 mL of DMF was added to a pre-dried 250 mL three-necked flask, followed by the prepared PT1N (1.5 g). Add 20 mL of a DMF solution containing NBS (1.6 g) dropwise while stirring in an ice bath, and react in the dark for 5 h. After the reaction was completed, a large amount of ice water was added, the filter cake was obtained by filtration, and vacuum-dried at 60° C. for 24 hours. The yield is 92%, and the product is a light green solid powder.
[0047] PT2N monomer synthesis. Add 30 mL of anhydrous tetrahydrofuran to a previously dried 250 mL three-neck flask, and then add excess magnesium powder (1.65 g). Add 2-bromothiophene (3.33mL) dropwise while stirring, the reaction emits a lot of heat, in order to prevent the temperature from being too high, cool with a cold water bath to control the temperature, and control the reaction to proceed steadily for 5h. The prepared Grignard reagent suspension was quickly transferred to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com