Thermal activation delayed fluorescence high-molecular compound and preparation method and application thereof
A high-molecular compound, thermal activation delay technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of low photoluminescence quantum yield and low efficiency of PLED devices, and achieve high glass transition temperature and thermal decomposition Effects of temperature, efficiency improvement, and good film formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
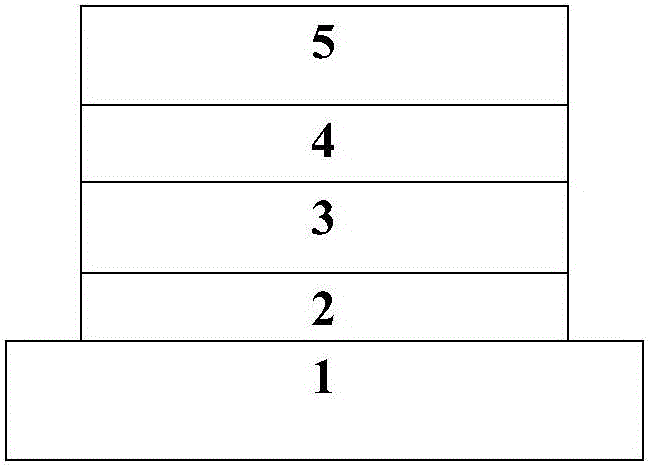
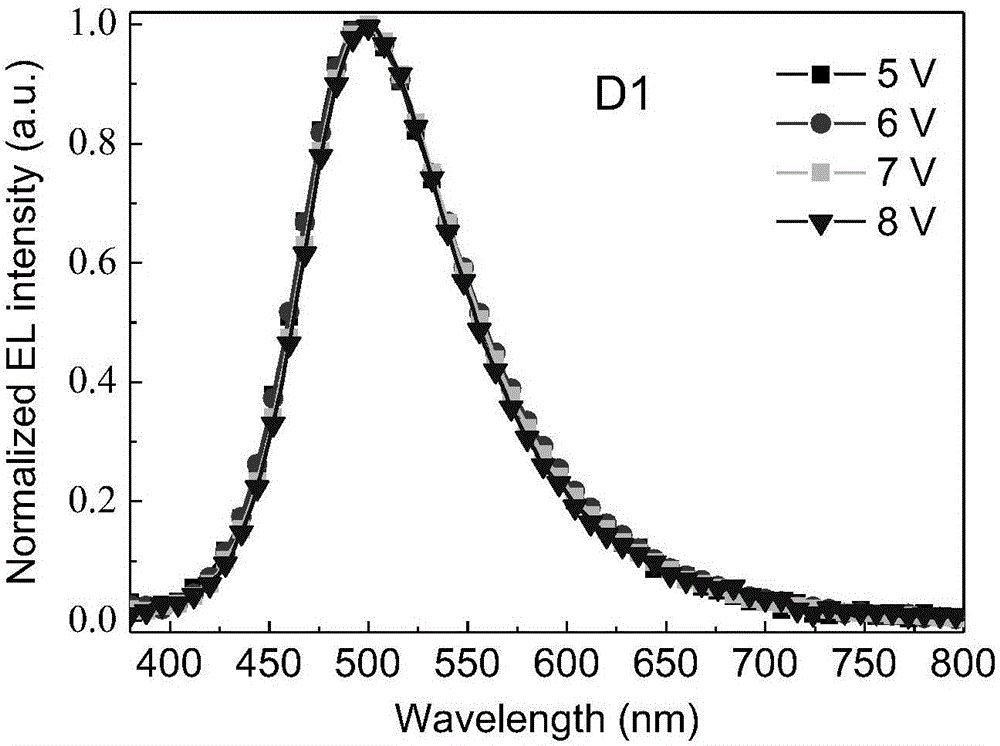
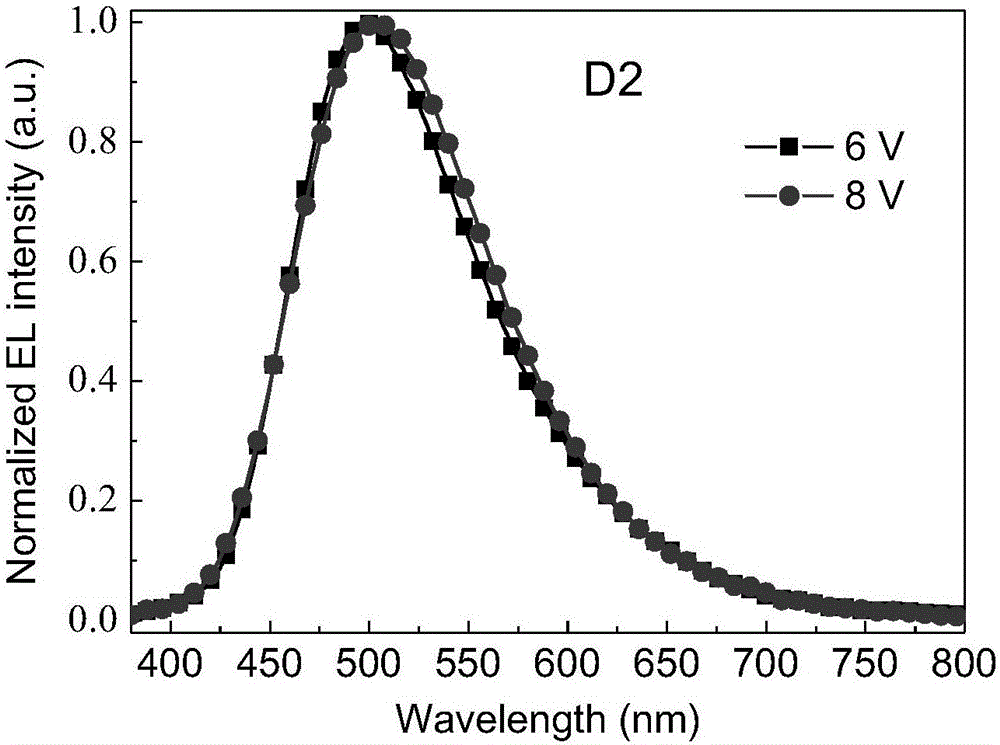
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of PCzDP series blue-green light polymers
[0033]
[0034] Under an argon atmosphere, weigh different amounts of M1, M2 and M3 in a 50mL Schlenk bottle, 0.44g potassium carbonate, 1mL tetrakistriphenylphosphopalladium toluene solution (0.02mmol / 10mL), 0.8mL methyltriphenylphosphopalladium The toluene solution of octylammonium tribromide (280mg / 16mL) and 4mL toluene were heated up to 110°C, stirred and reacted for 72 hours under the protection of argon, then cooled to room temperature, and 0.2g of phenylboronic acid was added under the argon atmosphere- The toluene solution was then reacted at 110°C for 12 hours, then lowered to room temperature, 3-4 drops of bromobenzene were added dropwise under an argon atmosphere, and the reaction was continued at 110°C for 12 hours. Cool to room temperature, settle with 250mL of methanol and 30mL of acetone mixed solvent, filter, dissolve the obtained polymer solid in dichloromethane, add 10mL of hydrogen pe...
Embodiment 2
[0044] Example 2: Synthesis of PCz-DPABP series green light polymers
[0045]
[0046] Under argon atmosphere, weigh different amounts of M1, M2 and M3 in a 25mL Schlenk bottle, 0.44g potassium carbonate, 1mL tetrakistriphenylphosphopalladium toluene solution (0.02mmol / 10mL), 0.8mL methyl triphenylphosphorous palladium Toluene solution of octylammonium tribromide (280mg / 16mL) and 4mL toluene, heated to 110°C, stirred and reacted for 72 hours under argon protection, then cooled to room temperature, added 0.2g of phenylboronic toluene under argon atmosphere solution, then reacted at 110°C for 12 hours, then lowered to room temperature, and added 3-4 drops of bromobenzene dropwise in an argon atmosphere, and continued to react at 110°C for 12 hours. Cool to room temperature, settle with 250 mL of methanol and 30 mL of acetone mixed solvent, filter, dissolve the obtained polymer in dichloromethane, add 10 mL of hydrogen peroxide (3%, v / v) aqueous solution, and stir at room temp...
Embodiment 3
[0052] Embodiment 3: Synthesis of PDF series blue-green polymers
[0053]
[0054] Under an argon atmosphere, weigh different amounts of M1, M2 and M3 in a 25mL Schlenk bottle, 0.44g potassium carbonate, 1mL tetrakistriphenylphosphopalladium toluene solution (0.02mmol / 10mL), 0.8mL methyl trioctyl Ammonium tribromide in toluene (280mg / 16mL) and 4mL of toluene, heated to 110°C, stirred for 72 hours under the protection of argon, then cooled to room temperature, and added 0.2g of phenylboronic acid toluene solution under argon atmosphere , and then react at 110°C for 12 hours, then lower to room temperature, drop 3-4 drops of bromobenzene in an argon atmosphere, and continue to react at 110°C for 12 hours. Cool to room temperature, settle with 250 mL of methanol and 30 mL of acetone mixed solvent, filter, dissolve the obtained polymer in dichloromethane, add 10 mL of hydrogen peroxide (3%, v / v) aqueous solution, and stir at room temperature for 5 hour, then dried with anhydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com