Organic solar cell material and preparation thereof
A technology of solar cells and bifunctional materials, applied in organic chemistry, circuits, photovoltaic power generation, etc., can solve problems such as small contact area, phase separation and cluster effect, and achieve thermal decomposition, improve photoelectric conversion efficiency, and increase absorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
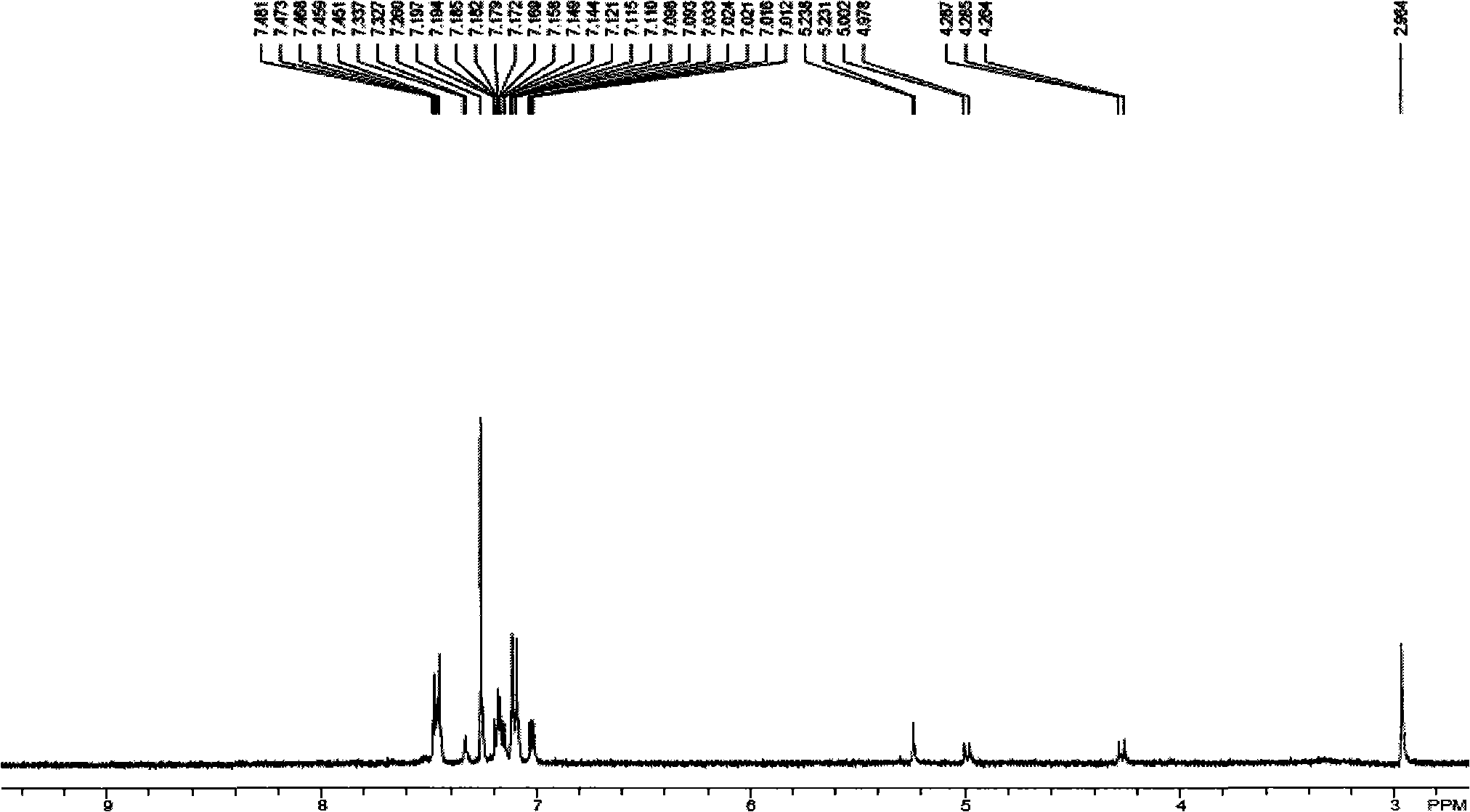
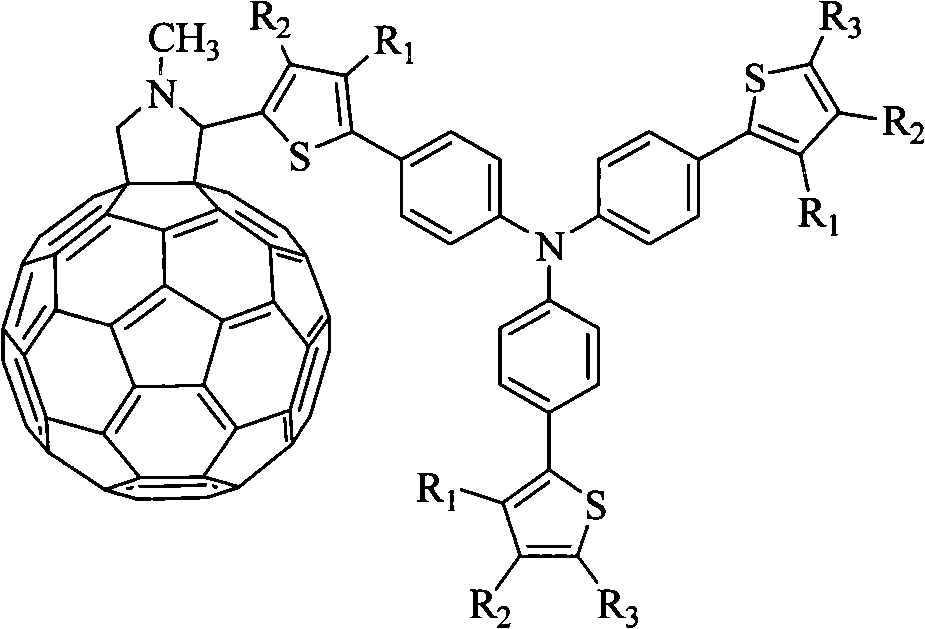
[0024] Embodiment 1, the preparation of N-methyl-2-thiophene-5-{4-bis[4-(2-thienyl)phenyl]amino}phenyl-fullerene pyrrolidine:
[0025] The synthetic route is as follows:
[0026]
[0027] ①Weigh 2.528g of polished magnesium bars and place them in a three-necked flask, add 15mL of distilled tetrahydrofuran THF, protect with nitrogen, add 5mL of 2-bromothiophene dropwise with a constant pressure funnel, and reflux for 1h after the dropwise addition to stop the reaction to obtain 2- Grignard reagent of bromothiophene, weigh 4.227g tribromotriphenylamine A and 0.135g PdCl 2 (PPh 3 ) 2 Add 30mL THF to a three-neck flask, stir and add Grignard reagent, protect with nitrogen, heat to reflux for 16h, add saturated ammonium chloride to quench the reaction, extract the product with chloroform, and dry over anhydrous sodium sulfate. Purified by column chromatography using n-hexane: dichloromethane volume ratio 3:1 as the eluent to obtain 3.48 g of yellow-green needle crystal compou...
Embodiment 2
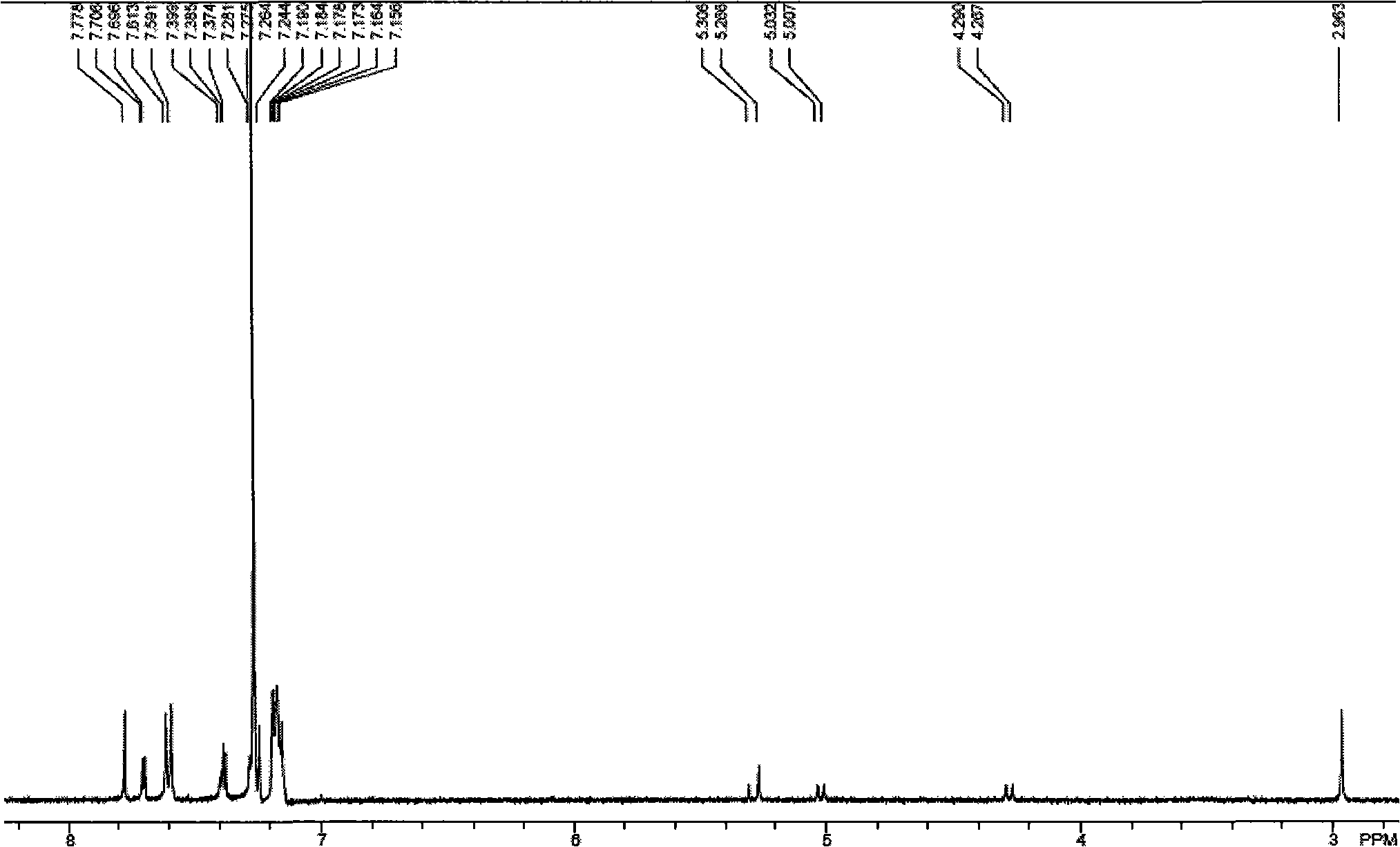
[0031]Example 2, Preparation of N-methyl-2-thiophene-5-{4-bis[4-2-(5-dicyanovinyl)thienylphenyl]amino}phenyl-fullerene pyrrolidine :
[0032] The synthetic route is as follows: the synthesis of trithiophene aniline is the same as that in Example 1.
[0033]
[0034] ① Weigh 0.613g of trithiophene aniline B into a three-necked flask, add 10mL of 1,2-dichloroethane, 2mL of DMF into it, protect it with nitrogen gas, and stir for half an hour under ice bath; start to drop the oxygen in the constant pressure funnel Phosphorus chloride 2mL, after the dropwise addition was completed, it was raised to room temperature and heated to reflux for 15 hours. Cool, pour into ice water, add 100mL saturated sodium acetate solution, add 50mL dichloromethane for extraction and separation, wash the organic layer several times with water, dry the organic layer with anhydrous sodium sulfate, use a mixed solvent of dichloromethane and ethyl acetate, dichloromethane The volume ratio of methane:e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com