Preparation method and application of triphenylamino metal organic framework compound capable of catalyzing carbon dioxide-epoxy compound cycloaddition
A technology of epoxy compounds and organic skeletons, applied in organic compound/hydride/coordination complex catalysts, organic chemical methods, catalyst activation/preparation, etc., to achieve good industrialization prospects, simple synthesis, and easy to be popularized and applied on a large scale Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
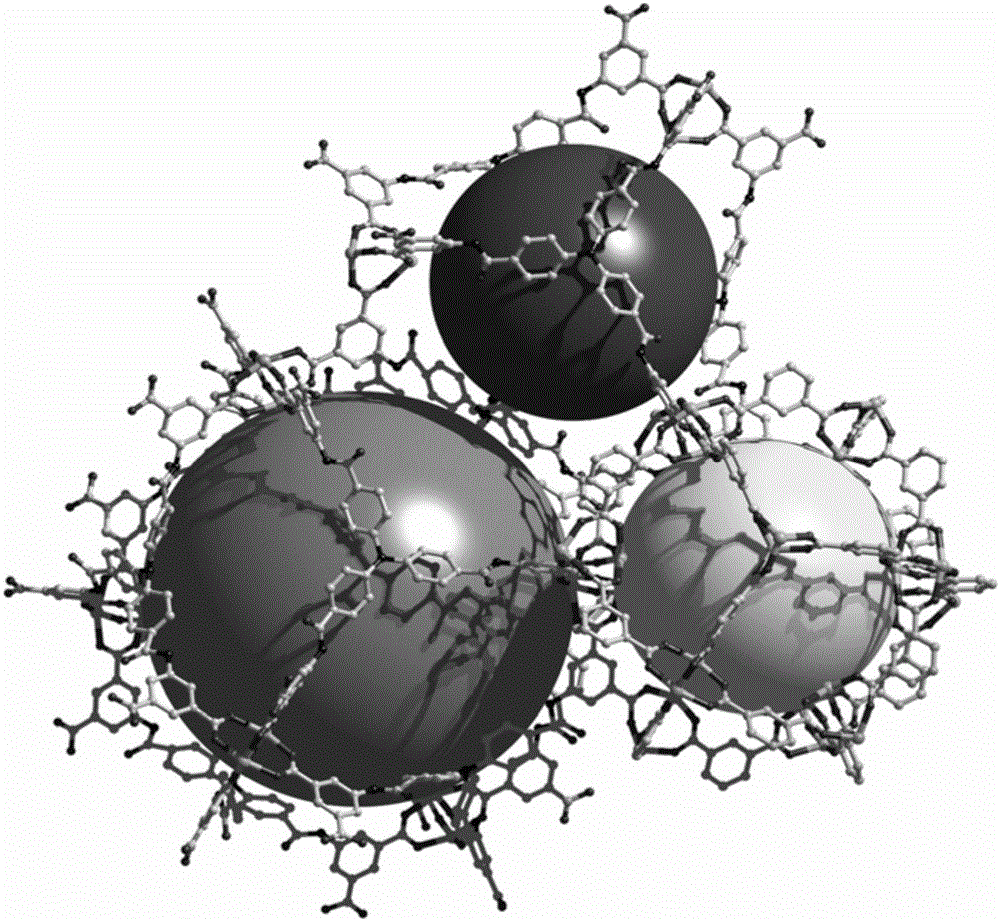
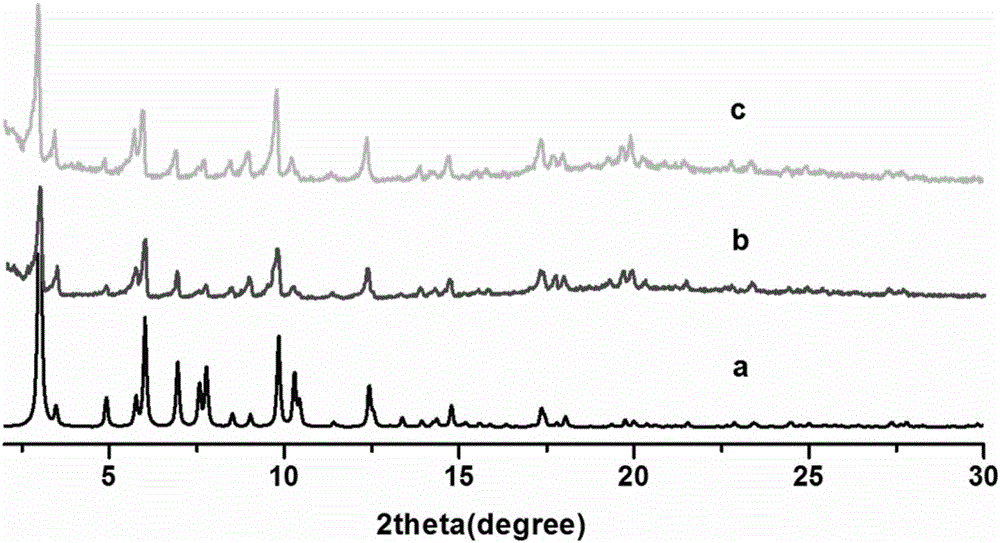
[0032] 5,5',5"-(4,4',4"-nitrilotritylamide)triisophthalic acid (17mg, 0.02mmol), Zn(NO 3 ) 2 ·6H 2 O (29.7mg, 0.1mmol) was dissolved in N,N'-diethylformamide (2mL) and ethanol (1mL) and stirred evenly, then the solution was placed in an oven, fired at 100°C for 72h, and the oven was closed. After cooling to room temperature, colorless to pale yellow cubic crystals were produced, filtered and dried to obtain the target material Zn-L with a yield of about 60%. Elemental analysis (%) for Zn 7 C 90 N 8 o 62.50 h 111 : C45.95, H 3.63, N 5.36%. Found: C 46.30, H3.70, N 5.19%. The structure of the obtained target material is as follows figure 1 As shown, the XRD pattern of the target material is shown in image 3 As shown, the thermal analysis diagram is shown as Figure 5 shown.
Embodiment 2
[0034] 5,5',5"-(4,4',4"-nitrilotritylamide)triisophthalic acid (17mg, 0.02mmol), Zn(NO 3 ) 2 ·6H 2 O (29.7mg, 0.1mmol) was dissolved in N,N'-diethylformamide (2mL) and ethanol (1mL) and stirred evenly, then the solution was placed in an oven, fired at 120°C for 72h, and the oven was closed. After cooling to room temperature, colorless to light yellow cubic crystals were produced, filtered and dried to obtain the target material Zn-L with a yield of about 56%.
Embodiment 3
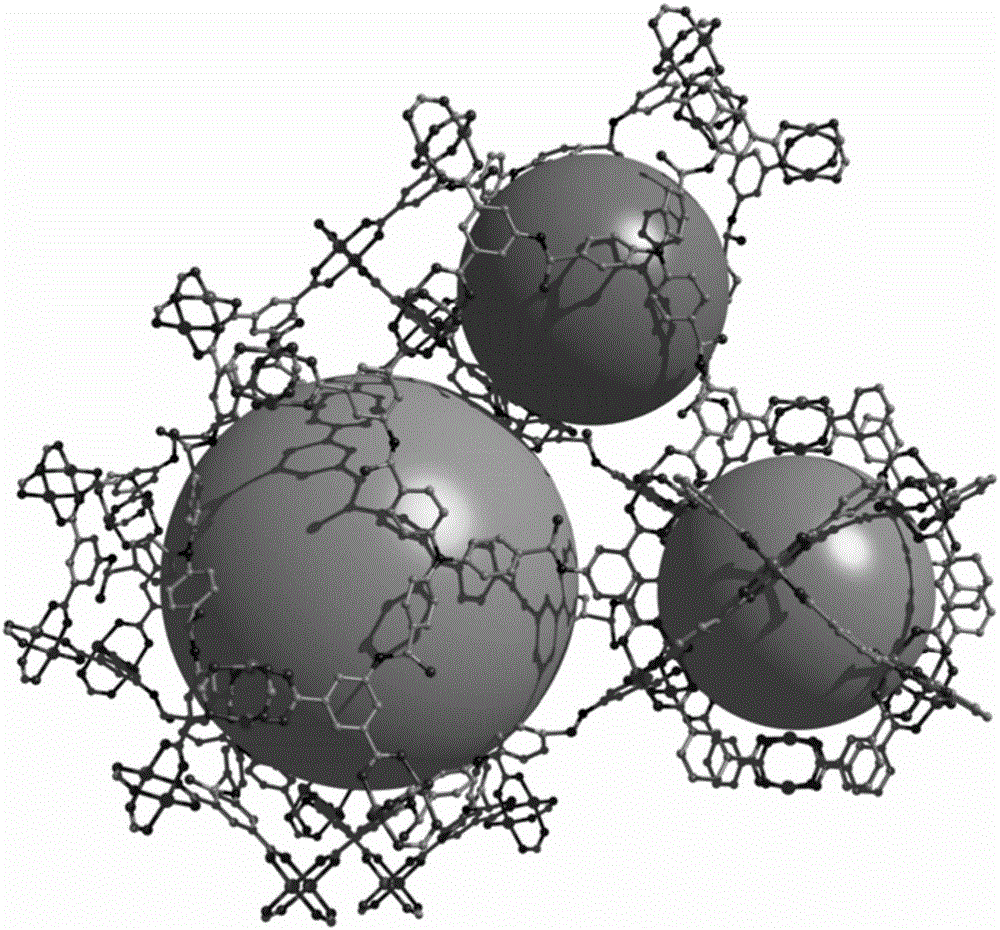
[0036] 5,5',5"-(4,4',4"-nitrilotritylamide)triisophthalic acid (17mg, 0.02mmol), Cu(NO 3 ) 2 ·3H 2 O (24 mg, 0.1 mmol) was dissolved in N,N'-dimethylformamide (2 mL) and concentrated HNO 3 (300 μL) after stirring evenly, take the solution and place it in an oven, bake it at 60°C for 72 hours, turn off the oven, cool to room temperature, green octahedral block crystals are produced, filter, and dry to obtain the target material Cu-L, The yield is about 70%. Elemental analysis (%) for Cu 6 C 90 N 8 o 42.75 h 73.50 : C46.36, H 4.11, N 6.69%. Found: C 47.24, H4.13, N 6.64%. The structure of the obtained target material is as follows figure 2 As shown, the XRD pattern of the target material is shown in Figure 4 As shown, the thermal analysis diagram is shown as Image 6 The adsorption curves of nitrogen and carbon dioxide are shown in Figure 7 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com