Compound for detecting secondary amine, and preparation and application thereof
A compound and secondary amine technology, applied in the field of compounds that can detect secondary amines, can solve the problems of cumbersome preparation steps in the detection process, cross-contamination between samples and reagents, unsuitable for emergency detection, etc., to achieve structural adjustment, sensitive action, structural easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The structure of the compound responsive to secondary amine is shown in formula (I)
[0055]
[0056] Synthesis of Compound 1 (R 1 = R 2 = R 5 =-(CH 2 ) 7CH 3 )
[0057]
[0058] 7-(8-(5-bromo-2-thienyl)-4,4-difluoro-4-boron-3a,4a-diaza-s-indene)-2-(4,4,5,5 - Preparation method of tetramethyl-1,3,2-dioxaborolan-2-yl)-9 octylcarbazole:
[0059] Weigh 531mg of 9-octylcarbazole-2,7-diboronic acid indyl alcohol ester (purchased from Beijing Shengweite Technology Co., Ltd.) and 350mg of 8-(5-bromo-2-thienyl)-4,4-difluoro- 4-boron-3a,4a-diaza-s-indene 2 (self-made in the laboratory, see below for the synthesis method) and 100mg of tetrakis-triphenylphosphopalladium were placed in a 100ml three-necked flask, deoxygenated and nitrogen-gassed, and then injected 30ml of tetrahydrofuran deoxygenated and 1ml of potassium carbonate (2M) aqueous solution were reacted for 24 hours, the mother liquor was spin-dried, and the crude product mixed with silica gel was sepa...
Embodiment 2
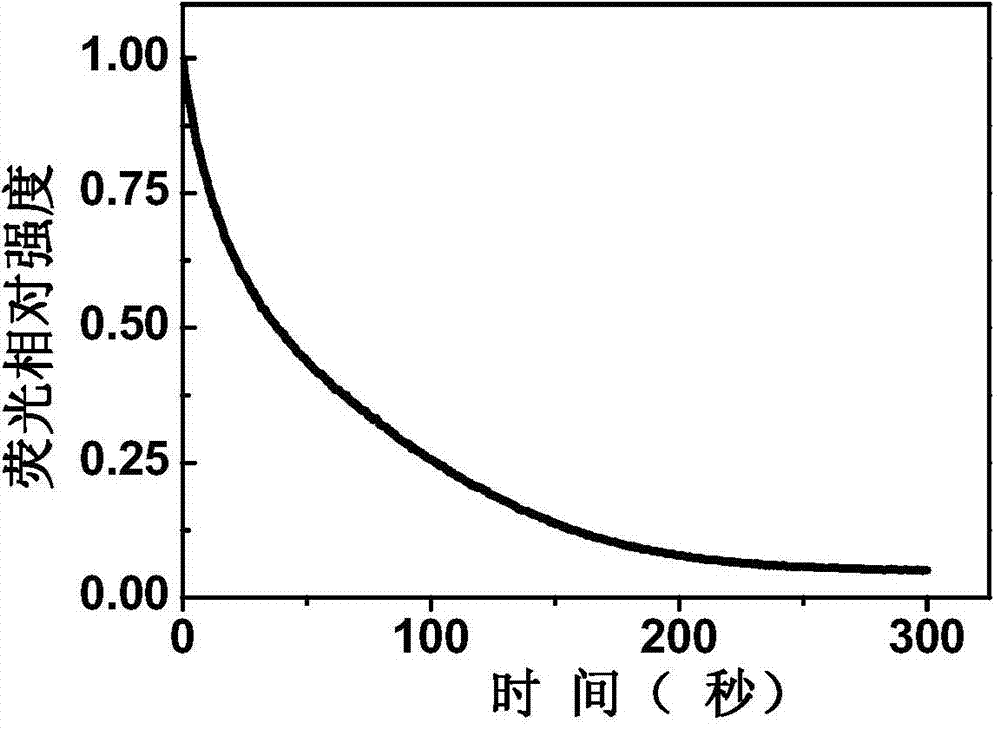
[0069] The sensing film based on compound 1 was prepared on the quartz substrate by pulling method. Put a drop of diisopropylamine on the bottom of the quartz cell, pad a ball of absorbent cotton above it to avoid direct contact with the sensing film, and seal the quartz cell with a cover. After placing the sensing film in a closed quartz cell, quickly measure the variation curve of the peak intensity and time at the maximum fluorescence emission peak. like figure 1 As shown, the intensity of the maximum absorption peak of its film was quenched by 95% within 300 seconds.
Embodiment 3
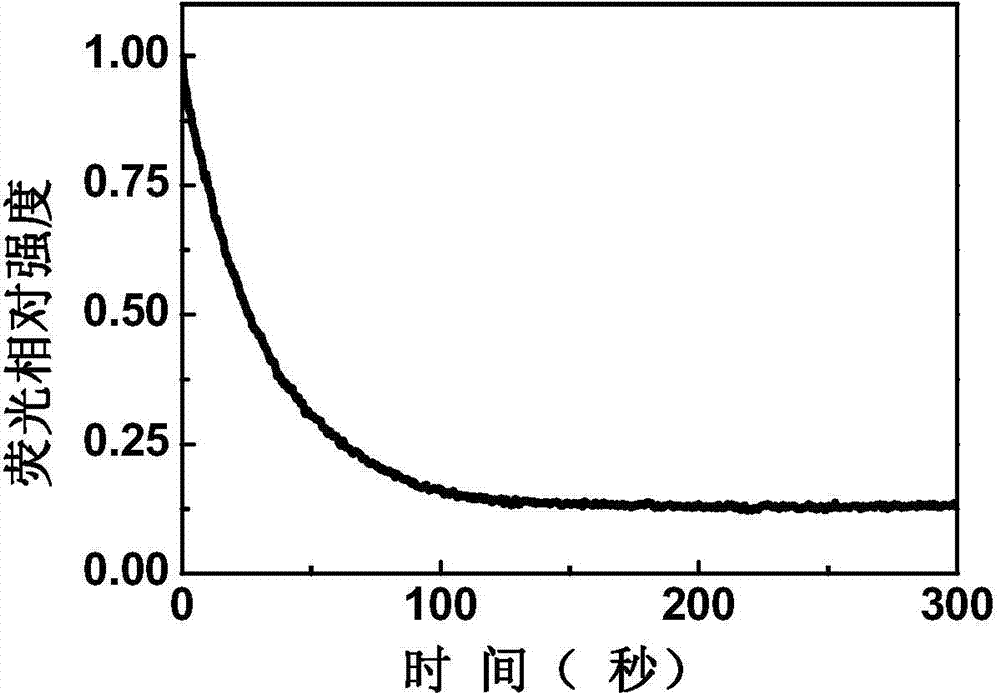
[0071] The sensing film based on compound 1 was prepared on the quartz substrate by pulling method. Put a drop of diethylamine at the bottom of the quartz cell, pad a ball of absorbent cotton above it to avoid direct contact with the sensing film, and seal the quartz cell with a cover. After placing the sensing film in a closed quartz cell, quickly measure the variation curve of the peak intensity and time at the maximum fluorescence emission peak. like figure 2 As shown, the intensity of the maximum absorption peak of the film was quenched by 89% within 300 seconds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com