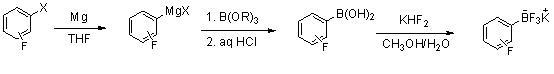Method for preparing potassium trifluoroborate series compounds
A technology of potassium trifluoroborate and compound, applied in the field of synthesis of potassium trifluoroborate compound, can solve the problem that potassium trifluoroborate series compounds are not suitable for large-scale production, the literature method does not propose a solution, and it is difficult to obtain high-quality products and other problems, to achieve the effect of excellent purity, simplified operation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
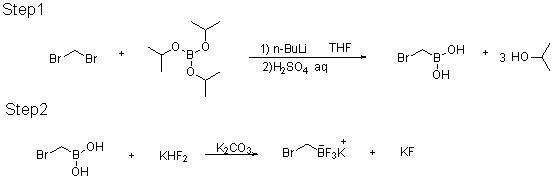
[0019]
[0020] At room temperature, a 500ml glass four-necked flask is equipped with mechanical stirring, a low temperature thermometer, a 200ml constant pressure dropping funnel, and an argon system; add 25g of dibromomethane, 27.05g of triisopropyl borate, and 200g of THF, and start stirring; Cool down with acetone dry ice freezing liquid; maintain the temperature of the kettle at -78°C, add 41.08g of n-butyllithium solution (2.5M / L) dropwise for about 2~4 hours; after the addition, keep the temperature of the kettle at -70~-78 Incubate at ℃ for 2-4 hours, quench with sulfuric acid aqueous solution, the reaction is completed, add 10% sulfuric acid aqueous solution dropwise to the reaction kettle to quench, adjust PH = 0 to 3; static layer, separate the water layer and extract with MTBE, the organic layer Put it into a 500ml four-necked bottle after merging, start stirring; add 44.94g KHF2 solid, 25g water, maintain the temperature of the kettle at 0-35°C, keep stirring...
Embodiment 2
[0022]
[0023] At a temperature of about 10-35°C, the Grignard reaction is initiated. Put 0.12mol of fluorobromobenzene (dissolved in 80mL ether) into a 500ml glass four-neck flask, and the amount of magnesium used is 1~1.5eq; slowly add the reaction solution dropwise to 0.26mol of B(OR)3 in ether solution Control the temperature at -70~-78°C; after the dropwise addition, control the temperature at about 0°C, add 10mL of 15% hydrochloric acid to adjust the pH value to about 1~2; separate the organic layer, and extract once with an equal amount of THF. The organic layers were combined, dried, the solvent was distilled off under reduced pressure, and beaten with diethyl ether to obtain 13.10 g of fluorophenylboronic acid, with a yield of 78%.
[0024] Dissolve 12.6 grams of fluorophenylboronic acid in 35 mL of methanol, add 25 grams of potassium bifluoride and 20 grams of water, after the addition is complete, stir for 3 to 12 hours; add K2CO3 to neutralize to pH = 7 to...
Embodiment 3
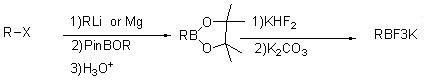
[0027]
[0028] Add 6.6g of 2-trifluoromethylfuran (0.0487mol) to a dry 100mL four-neck flask equipped with mechanical stirring and protected by argon (nitrogen is also acceptable) placed in a freezer, and 32ml of tetrahydrofuran is stirred to cool down ; Add n-butyllithium (19ml / 2.5M, 0.0485mol) dropwise to the reaction flask at 10~-18°C, after the addition is complete, keep it at -5~10°C for 1~3h; cool down to -72°C, and control it at -65℃~-78℃, start to add triisopropyl borate (13.8g, 0.073mol, 1.5eq) dropwise, keep warm for 2h after the dropwise addition; add 10ml MTBE to the reaction system, control the temperature below 15℃, add under stirring 1N HCl pH=6~7;
[0029] The aqueous layer was extracted with MTBE, the organic layers were combined, 11.4g of potassium bifluoride (0.146mol) and 4.2g of water were added under stirring at room temperature, and after stirring for 2-12 hours, potassium carbonate was added to adjust the pH to 7-9 and stirred for 1-3 , filtered, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com