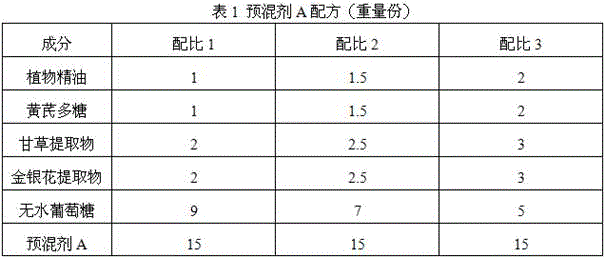
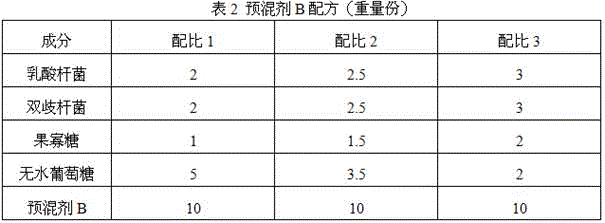
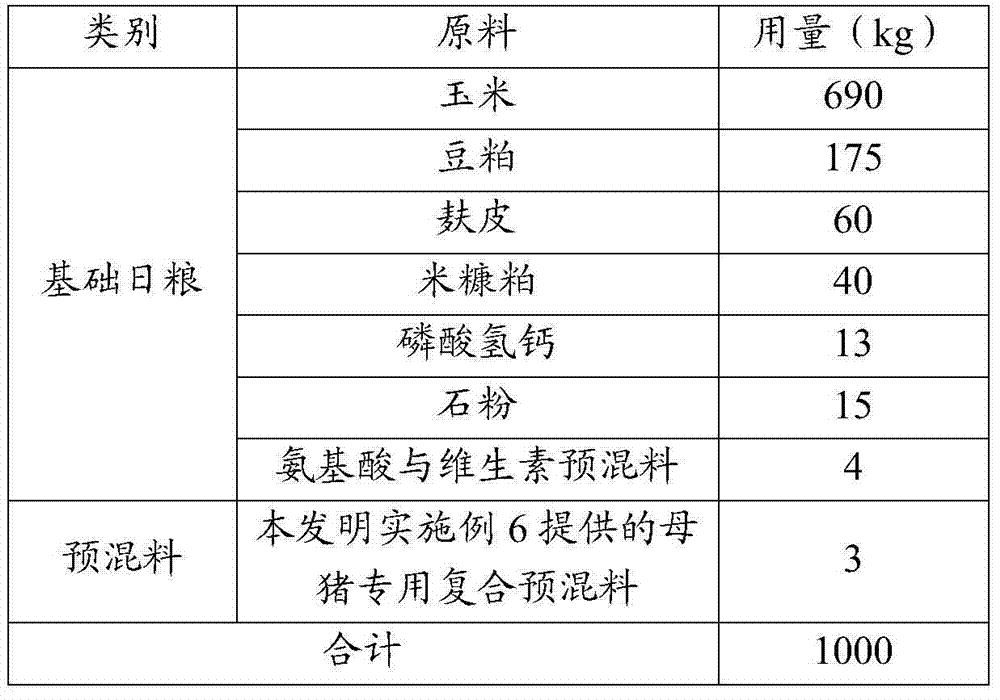
Patents
Literature
247 results about "Ferrous Fumarate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is an iron supplement used to treat or prevent low blood levels of iron (e.g., for anemia or during pregnancy).
Transdermal method and apparatus
Owner:ALDRED KATHERINE M
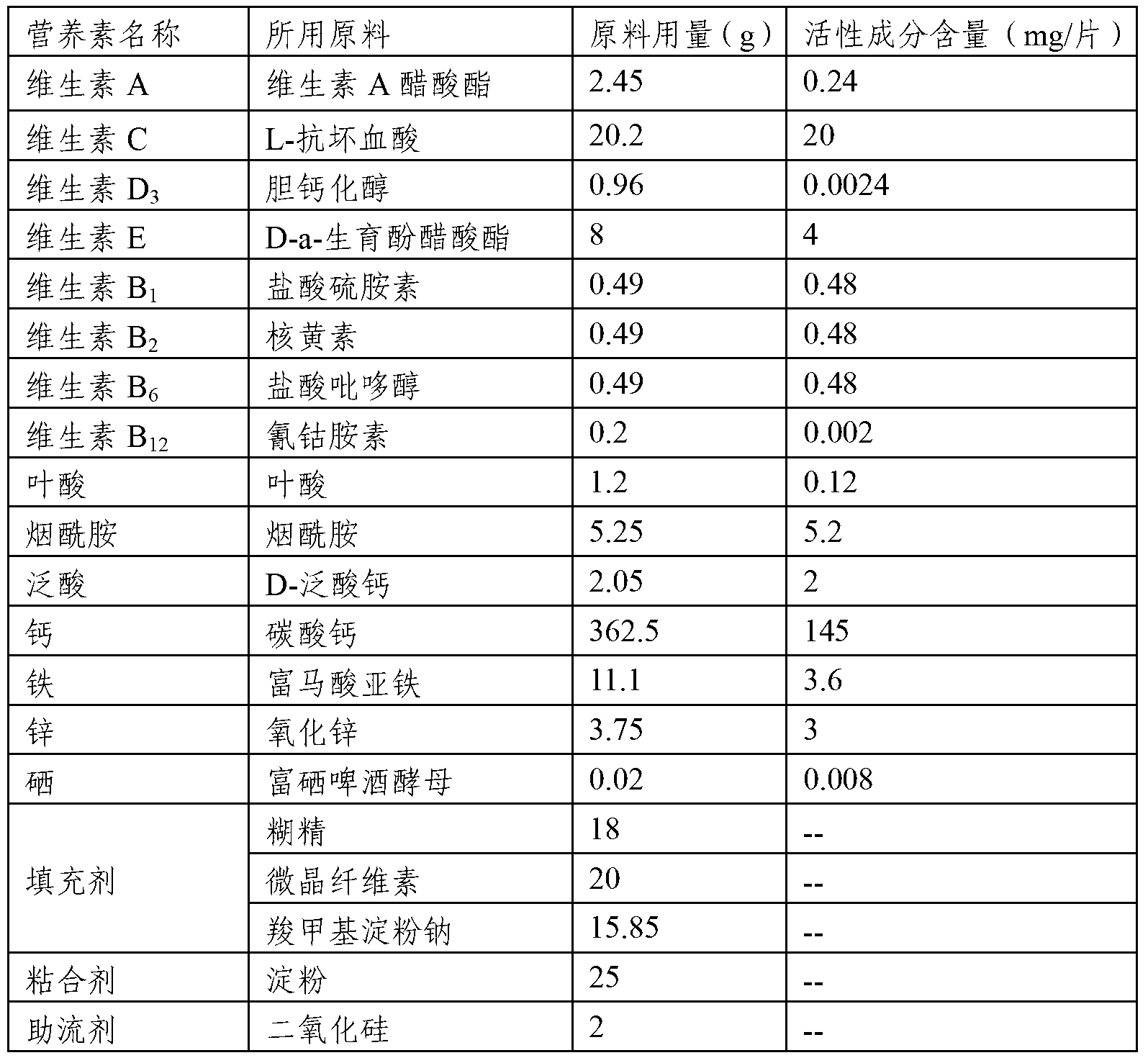
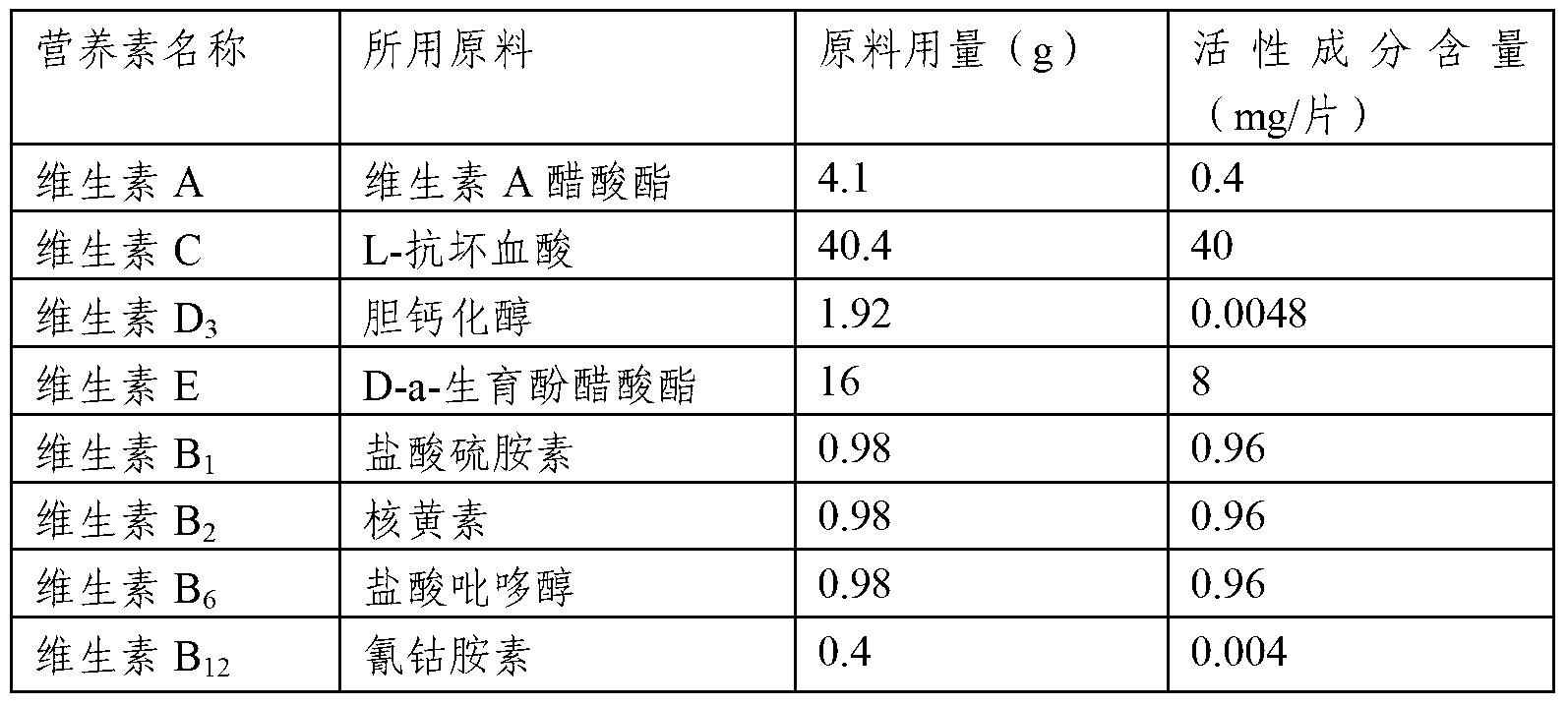
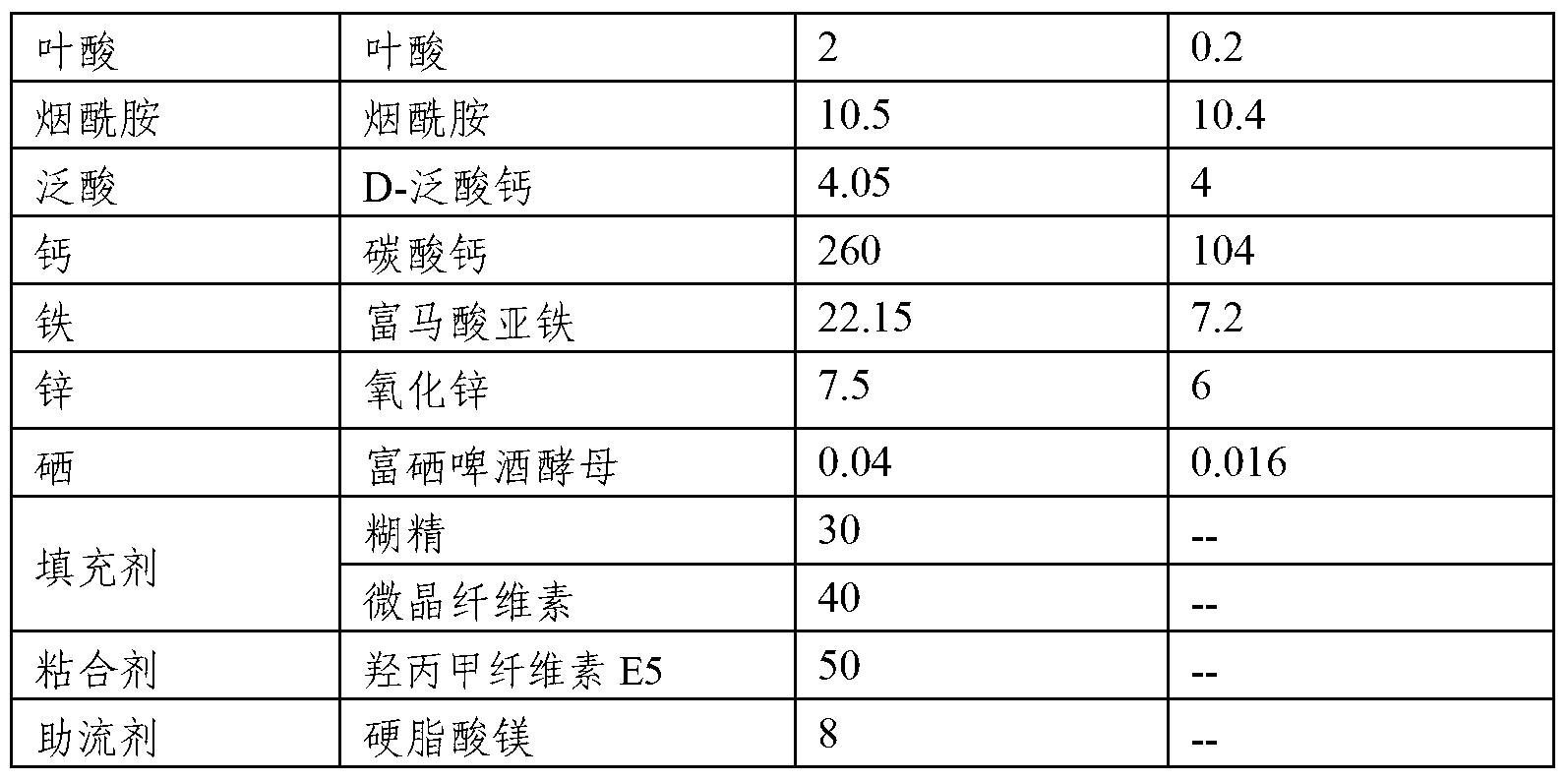
Composition comprising a plurality of vitamins and mineral substances
InactiveCN103284164AAvoid destructionIncrease mineralsFood preparationAdditive ingredientPantothenic acid
The invention relates to a composition comprising vitamins and mineral substances. The composition comprises the vitamins and the mineral substances, wherein the vitamins include vitamin A, vitamin C, vitamin D3, vitamin E, vitamin B1, vitamin B2, vitamin B6, vitamin B12, folic acid, nicotinic acid amide and pantothenic acid, and the mineral substances include calcium, iron, zinc, and selenium. According to the composition, the amounts of the vitamins and the mineral substances are reasonably proportioned strictly according to the needing amounts of a human body; the mineral substance iron is supplied by ferrous fumarate, so that the destroy on the active substances, such as vitamins, by the addition of the inorganic iron can be effectively prevented; the mineral substance selenium is provided by selenium enriched beer yeast, the toxic and side effects of chemical selenium (such as sodium selenite) on the human body and intestines and stomach stimulation are eliminated, and therefore the selenium can be absorbed and utilized by the human body more efficiently and safely.
Owner:HARBIN ZHENBAO PHARMA
Organic-inorganic composite trace element premix
The invention discloses premix of organic-inorganic complex trace element. The premix of the complex trace element is added with the organic trace element and the inorganic trace element, wherein the organic trace element is hydroxyl copper methionine, ferrous fumarate, hydroxyl iron methionine, hydroxyl zinc methionine, hydroxyl manganese methionine, selenomethionine and chromium picolinate. The inorganic trace element is anhydrous cupric sulfate, anhydrous ferrous sulfate, anhydrous zinc sulfate, anhydrous manganese sulfate and chromium nicotinate. The invention has the beneficial effects of greatly reducing the cost under no influence on the use effects, higher absorption and utilization and replacement of high copper, significant reduction on the discharge of the trace element, alleviating the adverse environmental impact from the culture production, effective protecting vitamin from being damaged, extending the product warranty period, increasing the myoglobin level, improving the carcass quality, enabling the animal fur to be ruddy and shiny, promoting the animal growth, improving the return of feed, promoting the body immunity and the anti-stress ability and promoting the reproductive performance of breeding stock and breeding bird.
Owner:广东省农业科学院畜牧研究所 +1
Transdermal method and apparatus
A transdermal patch for the treatment of iron deficiency including a drug reservoir layer containing an hematinic substance; a rate-controlling membrane secured to said reservoir layer; and a contact adhesive secured to said rate-controlling membrane, wherein said hematinic substance is selected from the class consisting of ferrous sulfate, ferrous lactate, ferrous iodide, ferrous gluconate, ferrous fumarate, ferrous citrate, ferrous carbonate saccharated, ferrous carbonate mass, ferronascin, ferroglycine sulfate, and ferrocholinate.
Owner:ALDRED KATHERINE M
Ammonium polyphosphate solutions containing multi-functional phosphonate corrosion inhibitors
InactiveUS6846437B2Reduce corrosionOther chemical processesLiquid fertilisersSuspending AgentsFerrous Gluconate
A corrosion-inhibited fire retardant composition is provided that comprises at least one ammonium polyphosphate, at least one suspending agent, at least one phosphonate selected from a group consisting of aminotri(methylenephosphonic acid), 1-hydroxyethylidene-1,1-diphosphonic acid, hexamethylenediaminetetra(methylenephosphonic acid), diethylenetriaminepenta(methylenephosphonic acid), salts thereof, and mixtures thereof and a corrosion inhibiting system. The corrosion inhibiting system is comprised of at least one corrosion inhibiting compound selected from a group consisting of azoles, insoluble ferric pyrophosphate, soluble ferric pyrophosphate, ferrous oxalate, ferric citrate, ferrous sulfate, ferric ammonium citrate, insoluble ferric orthophosphate, soluble ferric orthophosphate, ferric ammonium oxalate, ferric ammonium sulfate, ferric bromide, ferric sodium oxalate, ferric stearate, ferric sulfate, ferrous acetate, ferrous ammonium sulfate, ferrous bromide, ferrous gluconate, ferrous iodide, ferric acetate, ferric fluoroborate, ferric hydroxide, ferric oleate, ferrous fumarate, ferrous oxalate, ferrous oxide, ferric lactate, ferric resinate, and any combination thereof. Methods of making and using the same are also described. In addition, agricultural plant nutrients comprising the same are provided.
Owner:PERIMETER SOLUTIONS LP
Fire retardant compositions with reduced aluminum corrosivity
InactiveUS6905639B2Reduced-tendency to corrode various metalBroaden applicationFireproof paintsAntifouling/underwater paintsBiopolymerFerrous Gluconate
Corrosion-inhibited fire retardant compositions and methods of making and using the same are provided. The corrosion-inhibited fire retardant compositions are comprised of at least one fire retardant component, at least one biopolymer having a particle size diameter of less than about 100 microns, and a corrosion inhibiting system. The corrosion inhibiting system is comprised of at least one corrosion inhibiting compound selected from a group of compounds including azoles, insoluble ferric pyrophosphate, soluble ferric pyrophosphate, ferrous oxalate, ferric citrate, ferrous sulfate, ferric ammonium citrate, soluble ferric orthophosphate, insoluble ferric orthophosphate, ferric ammonium oxalate, ferric ammonium sulfate, ferric bromide, ferric sodium oxalate, ferric stearate, ferric sulfate, ferrous acetate, ferrous ammonium sulfate, ferrous bromide, ferrous gluconate, ferrous iodide, ferric acetate, ferric fluoroborate, ferric hydroxide, ferric oleate, ferrous fumarate, ferrous oxide, ferric lactate, ferric resinate and any combination thereof. In a specific embodiment, the corrosion-inhibited fire retardant composition includes a xanthan biopolymer.
Owner:PERIMETER SOLUTIONS LP
Intestinal tract health protection agent capable of reducing stress of weanling pigs as well as preparation method and use method thereof
InactiveCN106509457ALower pHTo promote metabolismAnimal feeding stuffAccessory food factorsDiseaseAdditive ingredient
The invention belongs to the field of feed additives and particularly discloses an intestinal tract health protection agent capable of reducing stress of weanling pigs as well as a preparation method and a use method thereof. The intestinal tract health protection agent consists of the following ingredients: plant essential oil, radix astragali polysaccharose, licorice root extracts, honeysuckle flower extracts, lactobacillus, bacillus bifidus, fructooligosaccharide, tryptophan, glutamine, arginine, ferrous fumarate, zinc lactate, selenium yeast, vitamin C, vitamin B12, folic acid, vitamin B6, citric acid, lactic acid, phosphoric acid, nucleotide, taurine, acid proteinase, alkali protease, alpha-amylase and anhydrous glucose. The intestinal tract health protection agent disclosed by the invention is capable of effectively relieving weanling stress of piglets, and preventing stress disease (common diarrhoea) caused by the stress factor.
Owner:河南飞默生物科技有限公司
Biological feed composition containing organic microelements, application of biological feed composition, special sow compound premix, and special sow feed
ActiveCN103783293AImprove the breeding rateImprove survival rateAnimal feeding stuffBiotechnologyFERROUS FUMARATE/IRON
The invention relates to the field of feed, and in particular relates to a biological feed composition containing organic microelements, an application of the biological feed composition, a special sow compound premix, and a special sow feed. The biological feed composition comprises the organic microelements and yeast metabolism products, wherein the organic microelements comprise copper glycinate, ferrous fumarate, zinc lactate, manganese methionine, selenium yeast and chromium nicotinate. The yeast metabolism products in the biological feed composition can be used for promoting the developing of synergistic effects of the various microelements; the utilization rate of the microelements is increased, the premonition and the immunity of sows are improved, and the hormone metabolism related to the sow reproduction is adjusted, so that the fecundity of the sows is improved; the special sow feed containing the biological feed composition containing the yeast metabolism products and the organic microelements can obviously improve the estradiol level and the luteinizing hormone level of the sows, so that the fecundity of the sows is improved.
Owner:SICHUAN ANIMTECH FEED CO LTD
Donkey-hide gelatin calcium composition and preparing process thereof
InactiveCN101279089AOrganic active ingredientsPeptide/protein ingredientsIron supplementPhosphopeptide
The invention relates to a combination of donkey-hid gelatin and calcium and a preparation method thereof, which pertains to the preparation field of medical nutrition. Iron-deficiency and calcium deficiency exist at the same time, when a person supplements the calcium in a large dosage, the calcium inhibits the body to absorb the iron elements and impacts the effect of blood enriching; in order to overcome the problem, the invention discloses a formula of the combination of donkey-hid gelatin and calcium; the formula not only comprises donkey-hid gelatin, calcium lactate, calcium gluconate, but also the iron-supplementing agent of ferrous fumarate, the calcium and iron absorption enhancer of casein phosphopeptide and excipients acceptable by pharmacology. The combination realizes the purpose of supplementing the calcium and the iron at the same time, thus having the advantages of reducing types of medicines taken by a patient, improving nutritional anemia, enhancing bone mineral density and reducing economic burden of the patient.
Owner:新疆华世丹药业有限公司
Preparation method of multifunctional konjac meal replacement powder
ActiveCN106819993ANutritional balanceReduce heatNatural extract food ingredientsFood ingredient functionsWhey protein powderMaltitol
The invention belongs to the field of foods and particularly relates to konjac meal replacement powder for assisting in reducing blood glucose, reducing blood fat and losing weight, and a preparation method thereof. The multifunctional konjac meal replacement powder is characterized by comprising the following components: konjac powder, tea polyphenol, oat powder, whey protein powder, xylooligosaccharide, vitamin, maltitol, maltodextrin, beta-cyclodextrine, zinc gluconate, ferrous fumarate and fruit and vegetable powder. The method has the beneficial effects that comprehensive and balanced nutrients are provided for a human body, and the meal replacement powder has the advantages of being low in heat and rich in dietary fiber, contains necessary proteins, amino acids, vitamins and microelements for supplementing the human body to maintain the normal function, and also has multiple healthcare functions and pharmacological effects, such as the effects of reducing blood fat, reducing blood pressure, reducing blood glucose and losing weight.
Owner:杭州梅之音生物科技有限公司
Cordyceps militaris polypeptide composition as well as preparation method and application thereof
The invention provides a cordyceps militaris polypeptide composition as well as a preparation method and an application thereof. The composition comprise the following medicinal and edible traditional Chinese medicinal materials in parts by weight: 10-45 parts of cordyceps militaris, 5-15 parts of oyster polypeptide powder, 1-5 parts of corn peptide powder, 12-43 parts of sheep kidneys, 4-12 parts of donkey genitalia, 20-28 parts of mulberries, 5-19 parts of endothelium corneum gigeriae galli, 2-10 parts of folia perillae acutae, 2-9 parts of lotus roots, 1-6 parts of hawthorn, 1-8 parts of cabbages, 13-28 parts of Chinese chives, 5-15 parts of pollen pini, 1-5 parts of coix lachryma-jobi kerne, 3-10 parts of tomatoes, 5-19 parts of dandelion, 9-20 parts of roses, 0.01-0.6 part of ferrous fumarate, 0.01-0.6 part of zinc lactate and 0.01-0.6 part of zinc lactate as well as bio-polypeptide. The composition provided by the invention can be applied to preparing health-care foods for improving skin moisture, alleviating asthenopia, dispelling chloasma and improving the sexual function or medicines for treating depression and benign prostatic hyperplasia.
Owner:田立平
Organic microelement premix for egg chicken
InactiveCN102138643AIncrease profitImprove immunityAnimal feeding stuffAccessory food factorsDiseaseNutritive values
The invention discloses an organic microelement premix for an egg chicken, which comprises methionine hydroxyl-copper, ferrous fumarate, methionine hydroxyl-iron, methionine hydroxyl-zinc, methionine hydroxyl-manganese, seleno methionine and chromium picolinate. The organic microelement premix is used for feeding the egg chicken; when in use, the premix is added into a complete feed in a proportion of 0.1%-0.15%. In the invention, the utilization rate of microelements is greatly improved; experiments prove that the utilization rate of organic microelements is improved by about 5 times compared with inorganic microelements; as absorption and utilization rates are improved, the waste of resources is reduced, the discharge of the microelements is obviously reduced, and adverse effects on the environment are relieved; moreover, the immunity of the organism is improved, the disease resistance is enhanced, and the mortality is reduced; therefore, the quality of an eggshell is improved, the eggshell is smooth, and has bright color, and the nutritive value of a chicken egg is improved.
Owner:胡晓军
Beauty dispersible tablet compound preparation for helping woman delay senescence and preparation method thereof
InactiveCN104621431AFood ingredient as antioxidantVitamin food ingredientsLycoperseneCALCIUM CARBONATE/MAGNESIUM CARBONATE
The present invention discloses a beauty dispersible tablet compound preparation for helping woman delay senescence and a preparation method thereof. The beauty dispersible tablet compound preparation includes the following active ingredients: collagen, a variety of antioxidants, composite vitamins, composite mineral substances and auxiliary materials. A variety of the antioxidants include grape seed extract (anthocyanin), lycopene, vitamin E and vitamin C. The composite vitamins include beta-carotene, vitamin A, vitamin D3, lutein, folic acid, vitamin B1, vitamin B2, vitamin B6 and vitamin B12 (1%). The composite mineral substances include zinc gluconate, calcium carbonate, magnesium carbonate, ferrous fumarate and selenium yeast. The auxiliary materials include an excipient, a disintegrating agent, a lubricant, a sweetener, a colouring agent and an essence.
Owner:JIANGSU ALAND NOURISHMENT
Composition of vitamin and mineral as well as application thereof in medicine for treating eczema
InactiveCN102342956AInorganic phosphorous active ingredientsDermatological disorderBeta-CarotenePantothenic acid
The invention belongs to the field of composite medicines of vitamins and minerals, and particularly relates to application of composition in the production of a medicine for the treatment of eczema. The composition comprises vitamin A acetate, beta carotene, vitamin D2, vitamin E, vitamin K1, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, biotin, folate, nicotinamide, calcium pantothenate, potassium chloride, a mixture of potassium iodide and lactose, sodium molybdate, calcium hydrophosphate, magnesium oxide, zinc oxide, ferrous fumarate, copper oxide, chromium trichloride, manganese sulfate, nickel sulfate, stannous chloride, 24% sodium selenate solution, sodium silicate, sodium vanadate and sodium chloride. The composition is suitable for various eczemas, and is particularly suitable for the treatment of eczema on hands and feet or cracking eczema with the efficiency of 97.9%.
Owner:王询亮
Extended cycle multiphasic oral contraceptive method
A multiphasic method of contraception that provides for sequentially administering to a female of child bearing age: (a) a Phase I composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 30 mcg of ethinyl estradiol for about 4 to about 7 days; (b) a Phase II composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 40 mcg of ethinyl estradiol for about 8 to about 16 days; (c) a Phase III composition containing a progestogen in an amount equivalent to about 0.5 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 30 mcg of ethinyl estradiol for about 4 to about 7 days; and (d) optionally, a Phase IV composition which is a placebo or a non-steroidal component, such as for example, ferrous fumarate, for about 2 to about 9 days, wherein the ethinyl estradiol equivalent amount of estrogen in the Phase II composition is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in each of the Phase I and III compositions. Preferably the sequential administration of the Phase I, II, and II compositions is repeated the day following the completion of the administration of the Phase III compositions to provide an extended cycle multiphasic oral contraceptive method.
Owner:APTALIS PHARMA
Preparation method of ovalbumin emulsion having double-shell structure
ActiveCN109247425AGood activity and stabilityImprove emulsion stabilityMulti-step food processesAnimal proteins working-upIon exchangeHigh pressure
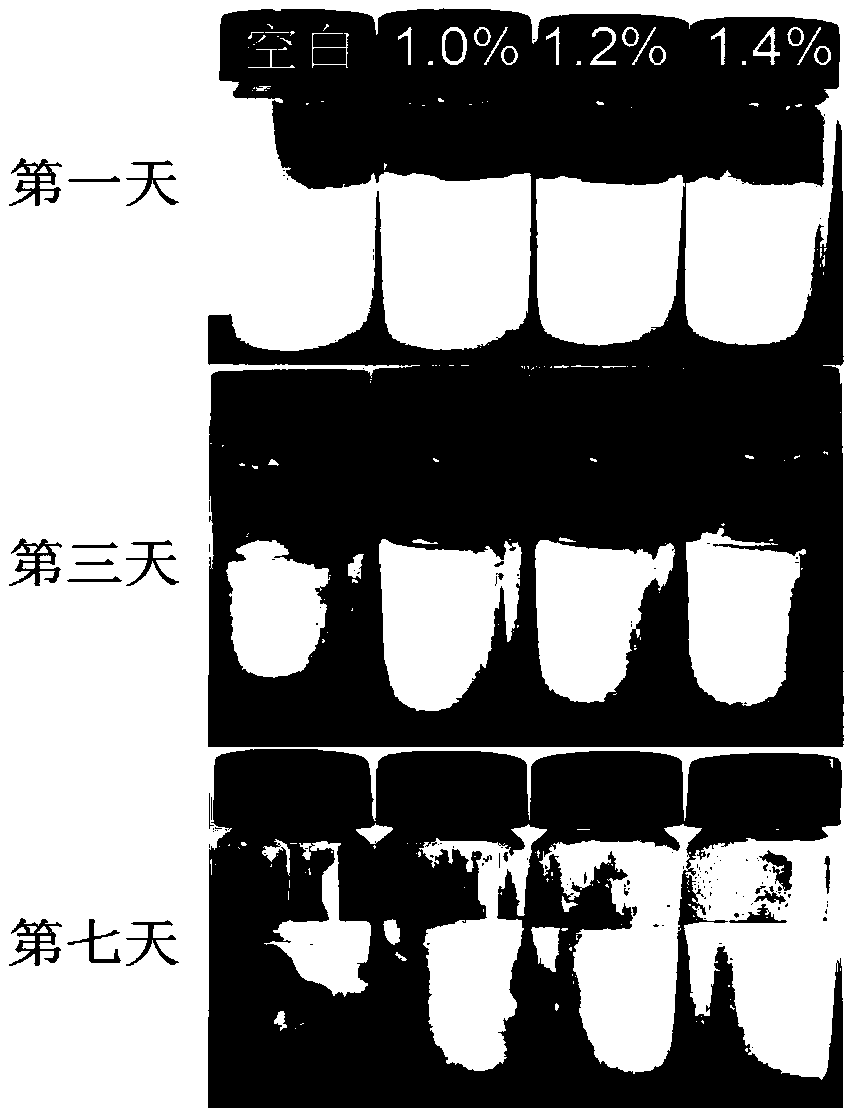
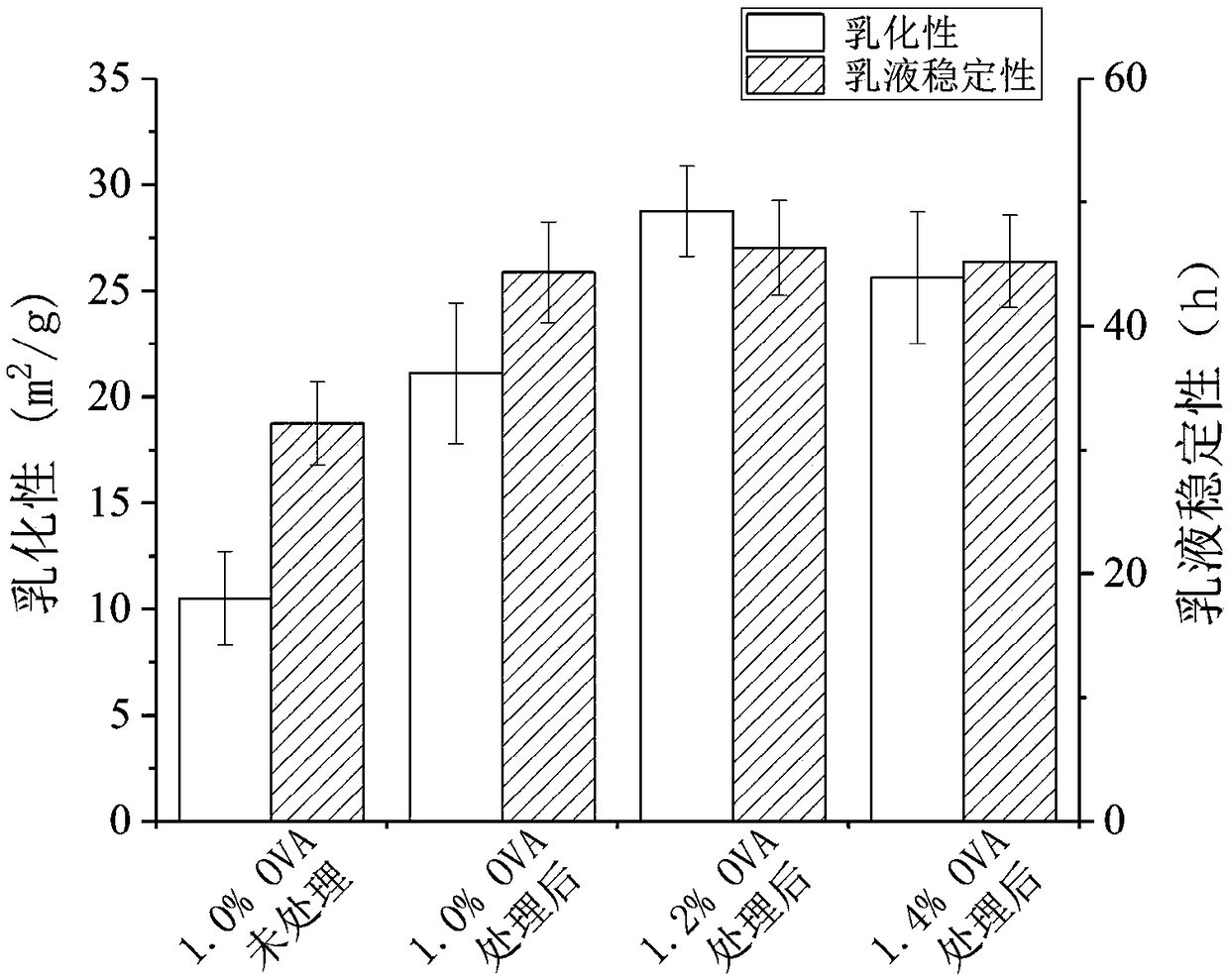
The invention discloses a preparation method of an ovalbumin emulsion having a double-shell structure. A purpose of the present invention is to solve the problems of to-be-improved shell-core stability and insufficient emulsifying property of the existing ovalbumin emulsion. The technical scheme comprises: (1) dissolving ovalbumin in water, carrying out magnetic stirring dissolving, adding inulin,and carrying out ultrasonic treatment to obtain an ovalbumin-inulin solution; (2) adding an oily core material to the ovalbumin-inulin solution, placing under a high-speed shearing device, shearing,and carrying out high-pressure micro-jet homogenizing a plurality of times to obtain an emulsion; (3) loading the iron ions of ferrous fumarate onto the surface of the emulsion droplet of the emulsionby using an ion exchange method to obtain a mixed solution, adjusting the pH value of the mixed solution, and carrying out centrifuging concentrating to obtain a concentrated emulsion; and (4) addingdisodium stannous citrate to the concentrated emulsion, and stirring at a low speed to obtain the ovalbumin emulsion. According to the present invention, the process is simple, the double-shell corestructure is stable, the emulsifying activity and the emulsifying stability of ovalbumin are substantially improved, and the ovalbumin emulsion has health effect, and further has good storage stability.
Owner:安徽荣达食品有限公司
Biological feed composition containing organic trace elements, application of composition, composite premix for growing and fattening of pigs and feeds specially for growing and fattening of pigs
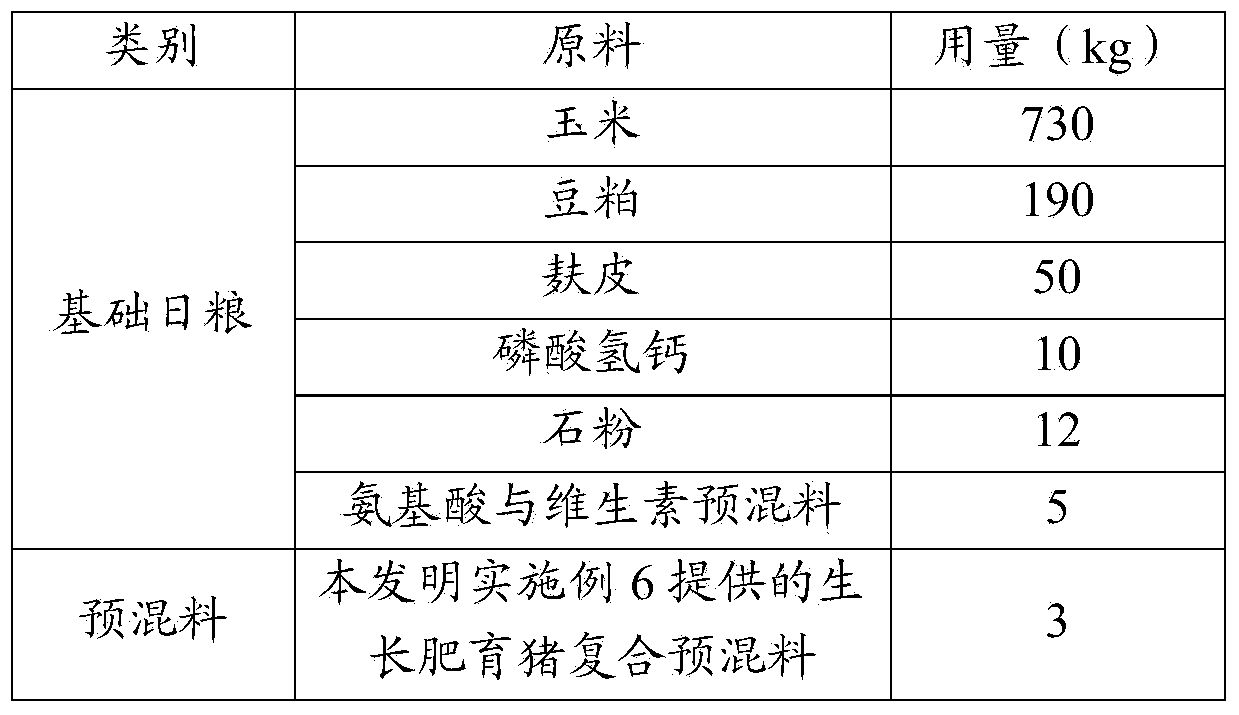
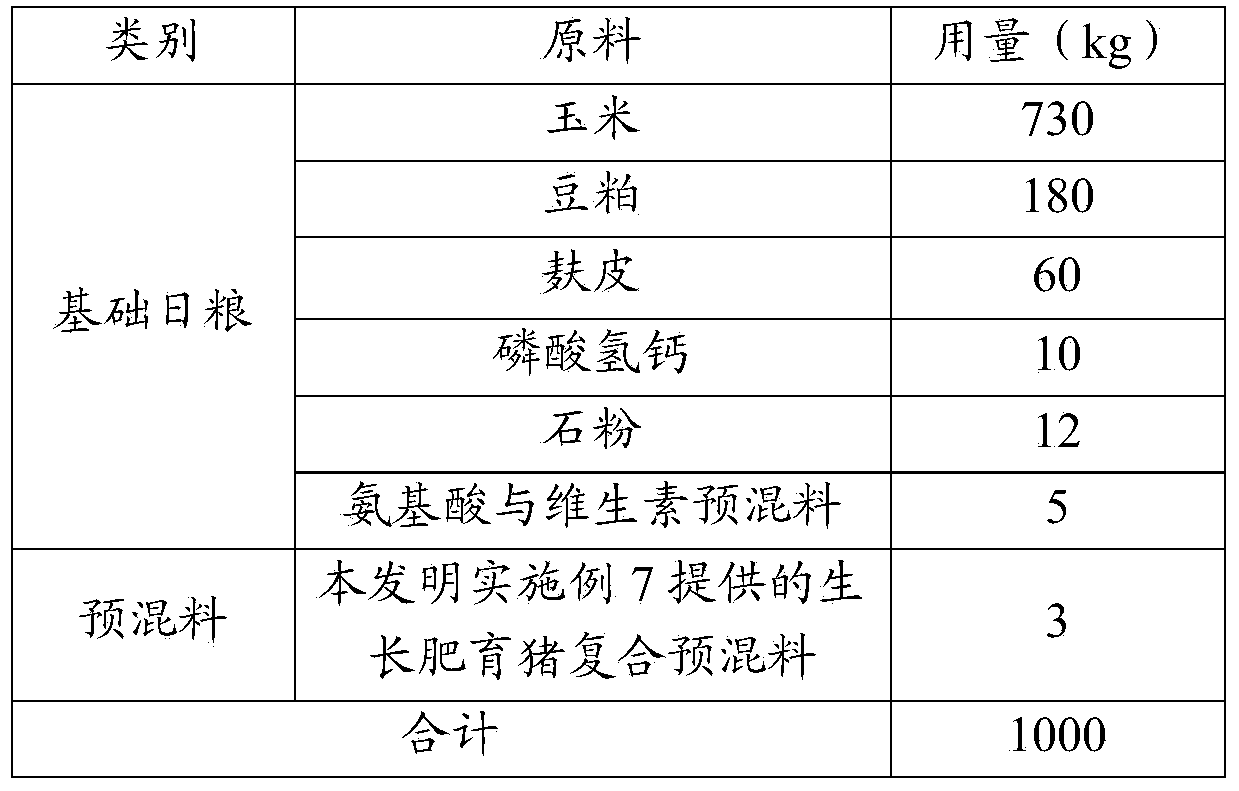
The invention relates to the field of feeds, and in particular relates to a biological feed composition containing organic trace elements, an application of the composition, a composite premix for the growing and fattening of pigs, and feeds specially for the growing and fattening of the pigs. The biological feed composition comprises organic trace elements and a yeast metabolic product, wherein the organic trace elements comprise copper glycine, ferrous fumarate, zinc lactate, manganese methionine, selenium yeast and chromium nicotinate. Due to the addition of the yeast metabolic product in the biological feed composition, a synergistic effect of all the trace elements can be achieved, and the utilization rate of the trace elements can be increased; by the feeds prepared from the biological feed composition, the lean meat percentage, loin eye area, color grade, muscle marble vein grade, muscle tenderness, intramuscular fat content and flavor grade of pork can be remarkably increased, the backfat thickness, the water loss rate and the drip loss can be remarkably reduced, and the carcass and quality of the pork can be remarkably improved.
Owner:SICHUAN ANIMTECH FEED CO LTD
Whey protein solid beverage
InactiveCN107258927AHigh organic ferrous contentPromote decompositionWhey manufactureSolubilityAdditive ingredient
The invention discloses a whey protein solid beverage. The whey protein solid beverage is prepared from the following raw materials: concentrated whey protein powder, inulin, resistant dextrin, a flavoring agent, vitamin A acetate, vitamin C, vitamin E acetate, thiamine hydrochloride, riboflavin, pyridoxine hydrochloride, vitamin B12, folic acid, calcium pantothenate, ferrous fumarate, zinc sulfate, maltodextrin, natural strawberry essence, silicon dioxide and stevioside. According to the whey protein solid beverage disclosed by the invention, the raw materials have high protein content and contain various types of vitamins, so that the absorption of proteins can be accelerated, the water solubility is relatively high and the mouthfeel is more fine and smooth. The whey protein solid beverage disclosed by the invention has the effects of supplementing physical ability, nutrients and various types of the vitamins, protecting eyesight and the like. The whey protein solid beverage has simple and reasonable raw material composition, natural components and a proper proportion; when being brewed with water, the whey protein solid beverage is more uniformly dispersed and is convenient for oral administration; the whey protein solid beverage is a solid beverage suitable for people.
Owner:深圳瑞森健康科技有限公司
Feed additive capable of saving feed protein consumption, production method and application thereof
ActiveCN103947840AIncrease production capacityImprove growth performanceAnimal feeding stuffAccessory food factorsFERROUS FUMARATE/IRONCitric acid
The invention belongs to the field of animal feed additives, and discloses a feed additive capable of saving feed protein consumption, a preparation method and an application thereof. According to the present invention, the feed additive contains lactic acid, citric acid, ferrous fumarate and a feed-grade carrier. The feed additive preparation method comprises: mixing lactic acid and a carrier, carrying out absorption of the carrier, adding citric acid and ferrous fumarate, and uniformly stirring and mixing to obtain the feed additive capable of saving feed protein consumption, wherein the feed additive capable of saving feed protein consumption is added to livestock and poultry breeding feeds, especially chicken breeding feeds, according to a certain ratio so as to reduce consumption of the protein raw material in the compound feed.
Owner:广州广牧丰生物技术有限公司
Health-care product for complementing multivitamins and mineral substances for pregnant women
InactiveCN107455758AStrong targetingPromote absorptionFood shapingFood ingredient functionsSucroseVitamin C
The invention discloses a health-care product for complementing multivitamins and mineral substances for pregnant women. 1000-1050g of the health-care product contains the following raw materials by weight: 1.337-2.0g of vitamin A acetic ester, 0.5-1.0g of vitamin D3, 0.42-0.56g of thiamine nitrate, 0.425g of lactoflavin, 0.475g of pyridoxine hydrochloride, 25-32.5g of vitamin C, 0.125-0.15g of folic acid, 3.75-4.2g of nicotinamide, 1.65-1.915g of calcium pantothenate, 499.97-749.99g of calcium carbonate, 12.25-28.6g of ferrous fumarate, 20.28-37.59g of zinc gluconate, 63.3-179.7g of starch, 0-94g of dextrin, 92-118g of cane sugar, 10-12g of carboxymethyl starch sodium, 10-10.5g of magnesium stearate and 20-21g of hydroxypropyl methylcellulose.
Owner:步源堂生物科技有限公司
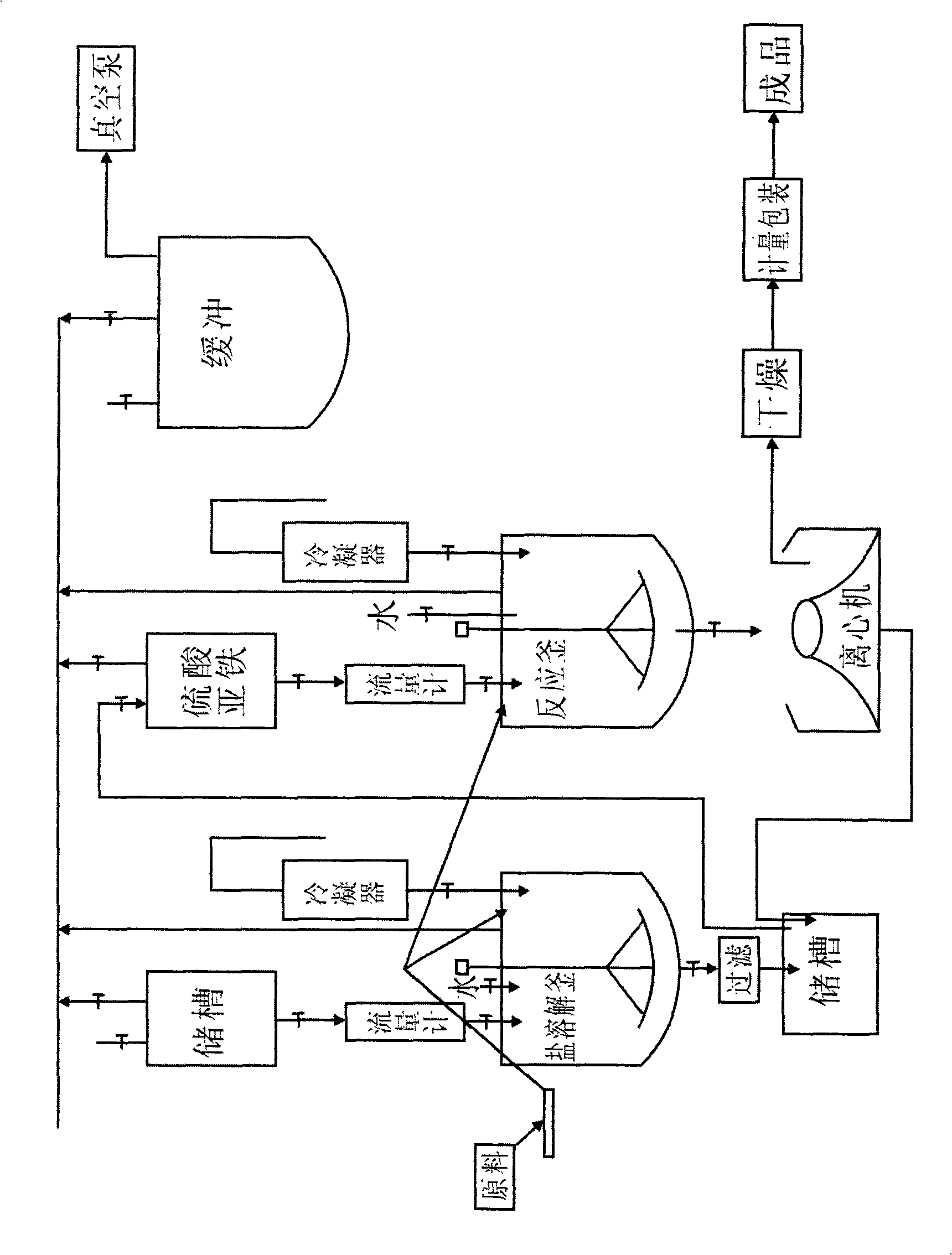
Process for producing ferrous fumarate
InactiveCN101643405AHigh purityHigh in ironAnimal feeding stuffCarboxylic acid salt preparationChemical reactionFERROUS FUMARATE/IRON
The invention discloses a process for producing ferrous fumarate. The process flow of the process comprises the following steps of: adding water in a salt dissolving kettle, and heating the water to atemperature of between 80 and 85 DEG C; adding fumaric acid and sodium carbonate in sequence; heating the mixture obtained by the previous step to a temperature of 100 DEG C; adding the mixture obtained by the previous step and ferrous sulphate in a reaction kettle; heating the mixture obtained by the previous step to the temperature of 100 DEG C for chemical reaction; discharging the resulting product and cooling resulting product; centrifugally separating the resulting product; drying the product of the centrifugal separation; measuring and packaging the product; and obtaining finished products. Due to the process, the production is free from 'three wastes' discharge, so that the environment is protected; the ferrous fumarate content of the product manufactured by the process is as highas 91.5 percent, the iron content of the product is 30 percent, the ferrous fumarate manufactured by the process can be widely used in animal feed additives in breeding production, is high in purity,iron content and absorption rate and low in cost and high in benefit, and can contributes to the sound and fast development of the state breeding industry.
Owner:SICHUAN ANIMTECH FEED CO LTD
Effervescence tablet beverage with fat-reducing function and its preparing process
ActiveCN1907058AWith weight loss functionHas the function of losing weight and anti-fatiguePre-extraction tea treatmentPlant ingredientsDiseaseAdditive ingredient
The invention discloses an effervescent beverage with fat-reducing function comprising main ingredients and auxiliary materials, wherein the main ingredients include (by weight portions) green apple essence 10-50, green tea extract 10-30, Hypericum perforatum extract 10-30, calcium gluconate 10-30, zinc gluconate 10-30, and ferrous fumarate 10-30, the auxiliary materials include (by weight ratio) lemon acid 5-20, sodium hydrogen carbonate 5-20, soluble starch 25-40, sweetener 3-8, flavoring agent 0.5-3, preservative agent 1-2. The preparing process consists of mixing main ingredients homogeneously, charging auxiliary materials, stirring homogeneously, moistening and granulating, sieving, charging disintegrating agent, lubricating agent, mixing homogenously, tabletting, dressing, checking and packaging.
Owner:GUIZHOU SHENQI PHARMA RES INST
Compound colla corii asini and dangshen and astragalus tablet and preparation method thereof
ActiveCN103040920AAppropriate useBeautyPharmaceutical delivery mechanismBlood disorderIron supplementMicrocytic anemia
The invention relates to a compound colla corii asini and dangshen and astragalus tablet. Alimentary anemia has two pathogenic factors including iron-deficiency anemia and megaloblastic anemia, but the existing nourishments and medicaments cannot get better efficacy on the two pathogenic factors simultaneously. The compound colla corii asini and dangshen and astragalus tablet is prepared from colla corii asini, a dangshen extract, an astragalus extract, ferrous fumarate, lactose, dextrin, microcrystalline cellulose, carboxymethyl starch sodium, copovidone, silicon dioxide, magnesium stearate and a transparent film coating agent according to the weight ratio of (337.5-412.5): (99-121): (76.5-83.5): (3.78-4.62): (267.3-326.7): (135-165): (90-110): (58.32-71.28): (54-66): (21.6-26.4): (9-11): (18-22). The invention further relates to a preparation method of the compound colla corii asini and dangshen and astragalus tablet. The compound colla corii asini and dangshen and astragalus tablet has most effects of traditional Chinese medicines and iron supplement Western medicines for enriching the blood, thereby being suitable for patients suffering from alimentary anemia and iron-deficiency anemia for use.
Owner:BY HEALTH CO LTD
Sunflower seed oil extracting agent
The invention discloses a sunflower seed oil extracting agent. The sunflower seed oil extracting agent comprises, by weight, 100 parts of ethanol, 20-30 parts of castor oil, 5-10 parts of sodium bicarbonate, 2-5 parts of amylase, 0.5-2 parts of ferrous fumarate and 0.02-1 part of citric acid. The sunflower seed oil extracting agent can effectively extract oil from a sunflower seed raw material against the characteristics of the sunflower seed oil, and the above solvent has no toxic side effects, so the recovery process requirements are not strict; and the solvent residual after the extraction is harmless to human bodies, and extracted oil meal can be directly used for feeding livestock and is harmless to the health of the livestock.
Owner:GUANGXI UNIVERSITY OF TECHNOLOGY
Additive premix with function of sow oxidative stress reduction
The invention discloses an additive premix with a function of sow oxidative stress reduction. The additive premix comprises a carrier and an additive composition, and the additive composition is prepared from 8-50mg / kg of 4',7-dyhydroxyl isoflavone, 10-25mg / kg of selenomethionine, 300-500mg / kg of ferrous fumarate, 100-400mg / kg of zinc glycinate, 10-1000KIU / kg of vitamin A, 30-70mg / kg of vitamin Eand 1-20mg / kg of vitamin C. The invention further provides a utilization method of the additive premix, adding the additive premix formed by a composition composed of biological activate substances ismore effective than adding any single component, and accordingly consumption of each additive is reduced, and cost saving is realized. By adoption of the additive premix, total antioxidant capacity of sow plasmas can be remarkably increased, sow lipid peroxidation degree is lowered, sow oxidative damage resistance is improved, and accordingly sow oxidative stress reduction is reduced.
Owner:广东新南都饲料科技有限公司 +2
Compound vitamin composition, and pharmaceutical preparation and application thereof
ActiveCN103877115AImprove stabilityOvercome stabilityMetabolism disorderEster active ingredientsVitamin CVitamin B12
Owner:海南新世通制药有限公司
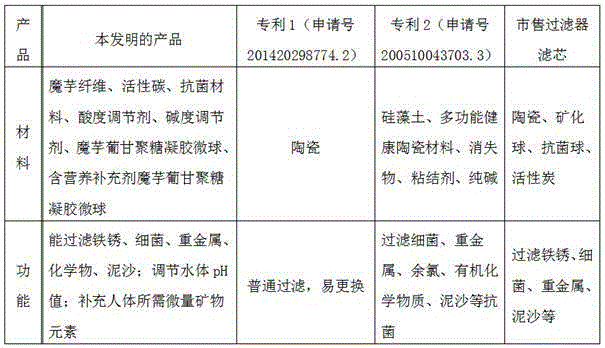
Konjac glucomannan aqua filter core and preparation method therefor
ActiveCN105032366AImprove adsorption capacityLong term releaseOther chemical processesWater/sewage treatment by substance additionFiberAdditive ingredient
The invention relates to a konjac glucomannan aqua filter core and a preparation method therefor. The preparation method for the konjac glucomannan aqua filter core comprises the steps of preparing konjac fibers, preparing konjac glucomannan gel microspheres, preparing nutritional supplement containing konjac glucomannan gel microspheres and preparing the filter core. The konjac glucomannan aqua filter core disclosed by the invention adopts konjac as a main raw material and plays a role by using the adsorption performance of the konjac fibers and the konjac glucomannan gel microspheres and the sustained release performance of the microspheres. The konjac fibers can be used for purifying water, and the konjac glucomannan gel microspheres can be used for filtering out most microorganisms in the water, so that microbiocides are not required to be used to a large extent; and the nutritional supplement containing konjac glucomannan gel microspheres can enable nutrients to be released in a long-term, stable and continuous manner so as to guarantee human body absorption efficiency. A nutritional supplement contains the ingredients, i.e., calcium carbonate, ferrous fumarate, zinc sulfate, sodium selenite, copper sulfate, manganese sulfate, chromium picolinate and the like and can be used for supplementing human required essential trace elements such as calcium, ferrum, zinc, selenium, copper, manganese, chromium and the like.
Owner:FUJIAN AGRI & FORESTRY UNIV
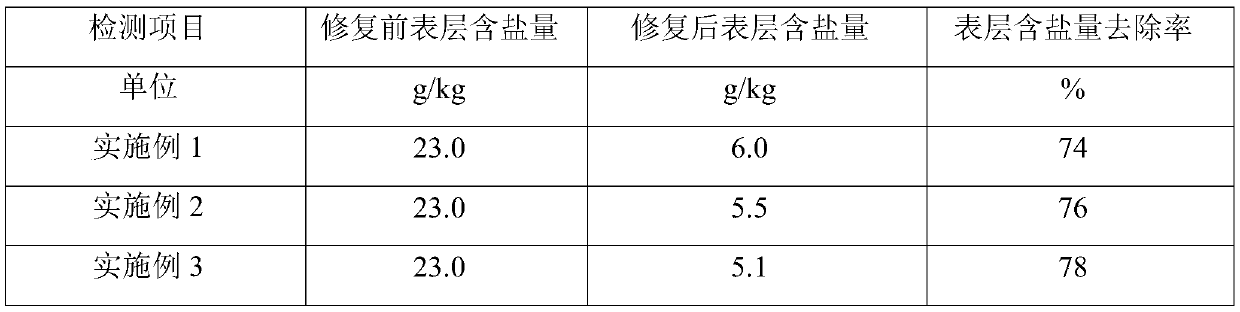
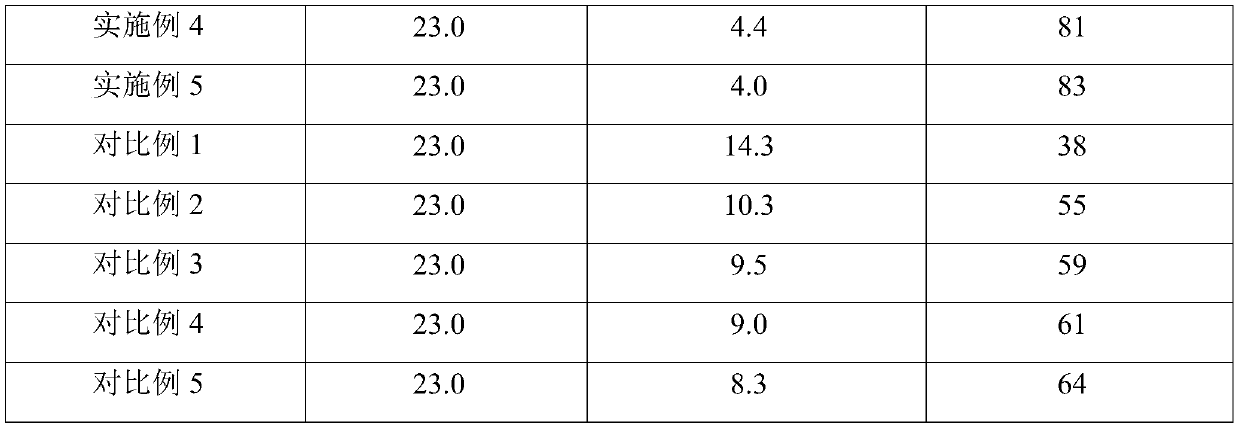
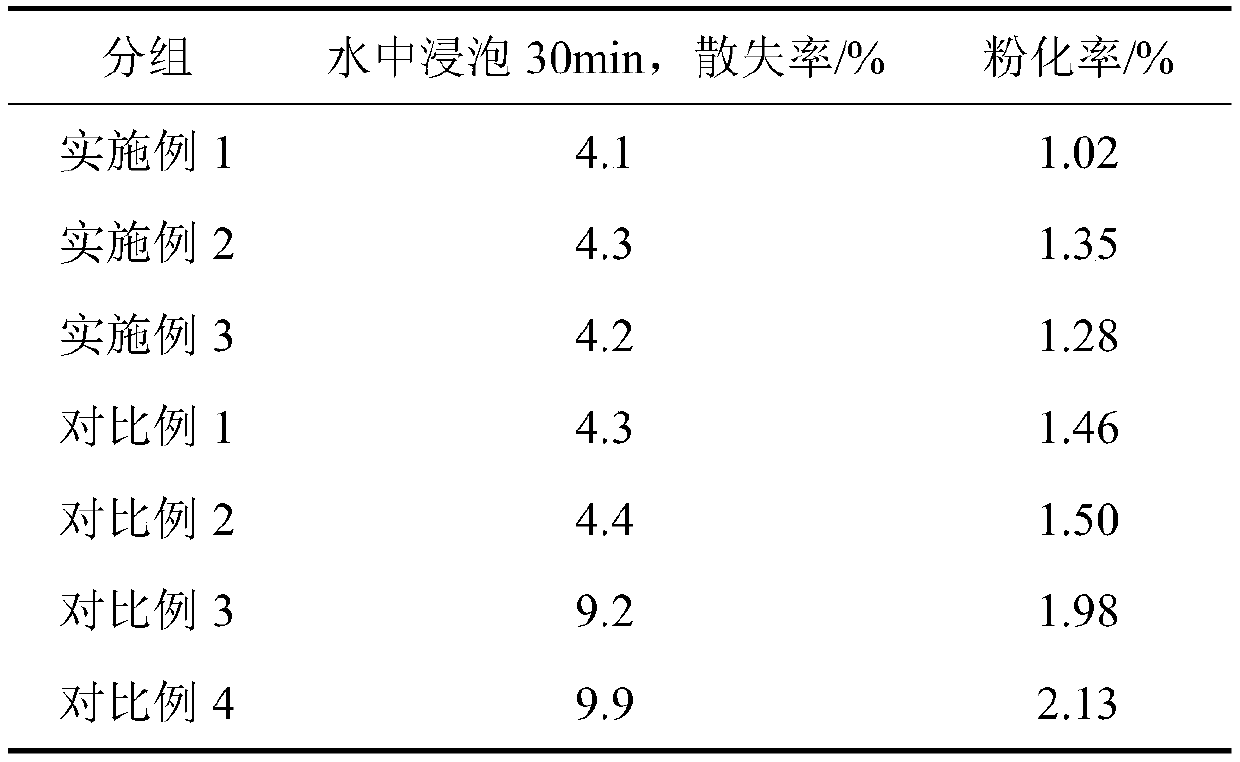
Method for repairing saline-alkali soil
ActiveCN109837090ASimple manufacturing methodEasy to operateContaminated soil reclamationOrganic fertilisersAlkali soilFERROUS FUMARATE/IRON
The invention provides a saline-alkali soil repairing agent, and the saline-alkali soil repairing agent is characterized by being prepared from the following raw materials in parts by weight: 25-35 parts of a ferrous fumarate organic acid copolymer, 5-10 parts of seaweed powder, 15-20 parts of a microbial fertilizer, 10-15 parts of calcium lignosulphonate, 5-10 parts of humic acid and 50-60 partsof water. The invention also provides a preparation method of the saline-alkali soil repairing agent and a repairing method for repairing saline-alkali soil by utilizing the saline-alkali soil repairing agent, by utilizing the embodiments of the present application, the saline-alkali soil can be safely, rapidly and efficiently repaired, the repairing cost is reduced, the production of secondary pollution is reduced, the yield of the saline-alkali soil is remarkably improved, and the quality of agricultural products is obviously improved.
Owner:广东宜瑞环境科技有限公司
Composite organic iron supplementing agent
InactiveCN105192318AGood effectReduce the cost of farmingAnimal feeding stuffCompound organicIron dextran
The invention provides a composite organic iron supplementing agent. The composite organic iron supplementing agent comprises the following raw materials by weight: 20 to 30 parts of ferrous fumarate, 10 to 20 parts of iron amino acid chelate, 5 to 10 parts of iron-dextran and 10 to 20 parts of ferrous lactate, wherein the iron amino acid chelate is one or a composition of a group consisting of ferrous lysinate, ferrous methioninate, ferrous glycinate and ferrous DL-threoninate. The composite organic iron supplementing agent is used as an animal iron supplementing agent and has good treatment and prevention effects on iron-deficiency anemia caused by chronic hemorrhage, malnutrition, pregnancy, growth and development periods, etc.
Owner:南宁市泽威尔饲料有限责任公司
Good water-resistant feed for river crabs and preparation method thereof
InactiveCN109717329AImprove water resistanceIncrease appetiteFood processingClimate change adaptationPullulanPorphyrin
The present invention discloses a good water-resistant feed for river crabs. The good water-resistant feed is prepared from fish meal, soybean meal, rapeseed meal, wheat flour, wheat gluten, corn protein powder, food attracting powder, a zinc ion-porphyrin nanocomplex, beta-D-mannuronic acid, bamboo salt, pullulan, calcium dihydrogen phosphate, a membrane coating material, multivitamins and ferrous fumarate. The present invention also discloses a preparation method of the good water-resistant feed for the river crabs. The good water-resistant feed is stable in raw material source, the materials cooperate with each other, complementary functions between the nutrients are exerted, the good water-resistant feed has advantages of comprehensive nutrition, good water resistance, high feed utilization rate and good attracting property, significantly improves feed intake, digestibility, immunity and disease resistance of the river crabs, reduces use of antibiotics, promotes growth and development of the river crabs, and effectively improves economic benefits of river crab farming.
Owner:周爱民
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com