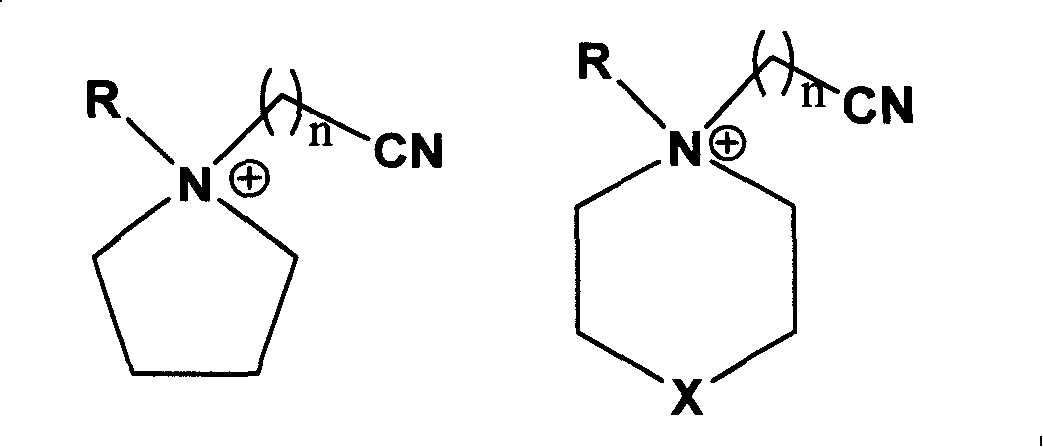
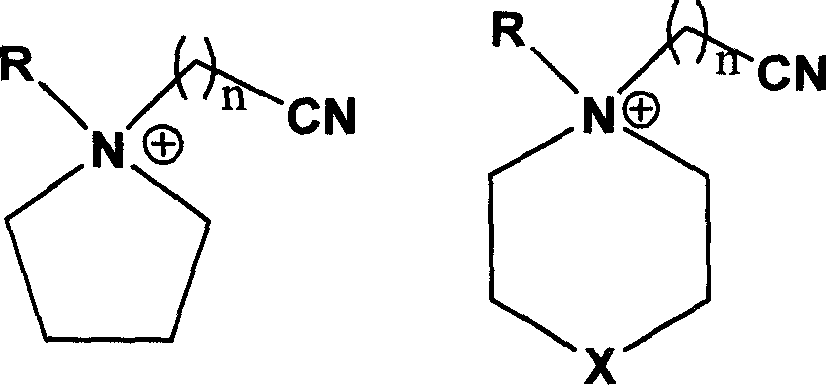
Ionic liquid with high electrochemical stability and preparing method thereof
An ionic liquid and electrochemical technology, applied in the direction of organic chemistry, can solve the problems of low electrochemical stability and hinder the application of ionic liquid, and achieve the effect of good electrochemical stability, high refractive index, and wide electrochemical window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of N-methyl-N-butyronitrile pyrrolidine chloride salt
[0039] Weigh 21.30g (0.25mol) of N-methylpyrrolidine and dissolve it in 50ml of absolute ethanol. After stirring at room temperature to make it fully mixed, add 31.1g of chlorobutyronitrile (0.30mol) to the above solution, and at 70°C The reaction was continued for 48 h under magnetic stirring, the solvent ethanol and other volatile components were removed by rotary evaporation, 40 ml of ethyl acetate was added for recrystallization, the resulting white solid was washed three times with anhydrous ether, filtered with suction, and the solid was evacuated at 100 °C for 8 Hours, the ionic liquid N-methyl-N-butyronitrile pyrrolidinium chloride was obtained with a yield of 88.4%.
Embodiment 2
[0041] Synthesis of N-methyl-N-butyronitrile pyrrolidine bis(perfluoroalkylsulfonyl)imide salt
[0042] Weigh 18.9 g (0.1 mol) of N-methyl-N-butyronitrile pyrrolidinium chloride salt and dissolve it in 50 ml of distilled water, and add 43.0 g (0.15 mol) of bis(perfluoroalkylsulfonyl)imide lithium salt under stirring at room temperature , after reacting for 4 hours, let it stand, separate layers, separate the lower oil phase with a separatory funnel, wash with distilled water for 3 times, and vacuumize at 100 ° C for 4 hours to obtain a colorless liquid N-methyl-N-butyronitrile Pyrrolidine bis(perfluoroalkylsulfonyl)imide salt, the yield is 78.2%, and the electrochemical window is 5.4V.
Embodiment 3
[0044] Synthesis of N-methyl-N-butyronitrile pyrrolidine tetrafluoroborate
[0045]Weigh 18.9 g (0.1 mol) of N-methyl-N-butyronitrile pyrrolidine chloride salt and dissolve it in 50 ml of absolute ethanol, add 16.5 g (0.15 mol) of sodium tetrafluoroborate under stirring at room temperature, and continue stirring for 48 hours. After aging and filtering, the filtrate was evaporated to dryness to obtain white solid N-methyl-N-butyronitrile pyrrolidine tetrafluoroborate with a yield of 86.1% and an electrochemical window of 5.3V.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com