Preparation method of nitrosyl sulfuric acid and method for separating sulfuric acid and phosphoric acid in industrial waste acid
A technology of nitroso sulfuric acid and nitric acid, which is applied in the preparation of alkali metal sulfites, chemical instruments and methods, phosphorus compounds, etc. The effect of reducing emissions and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
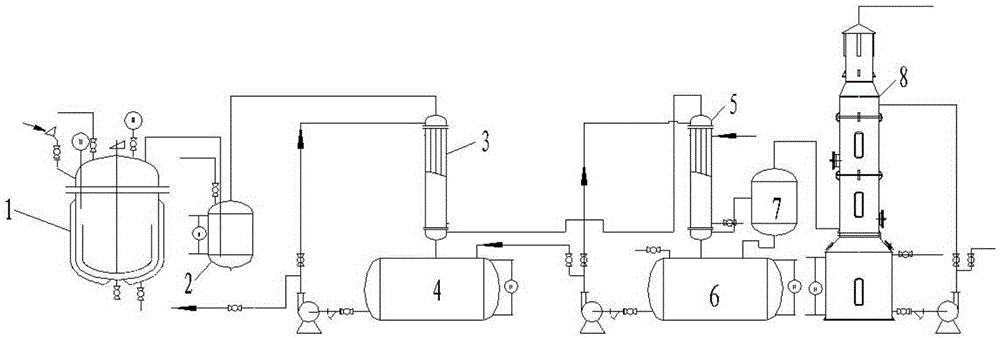
[0056] Step (1): Add 8kg of sulfur and 50kg of sulfuric acid into a 250L reaction kettle, heat up to 200°C for 1.5h, and the sulfur dioxide gas generated is connected to the concentrated sulfuric acid drying kettle through the pipeline for drying;
[0057] Step (2): the dried sulfur dioxide imports in the two-stage series falling film absorption tower, reacts and absorbs through the mixed nitric acid solution B, and the mixed nitric acid solution B is that 96.5kg mass fraction is 98wt% nitric acid and 546.8kg mass fraction is 98wt % of the sulfuric acid mixed solution (15wt% nitric acid and 85wt% sulfuric acid), mixed nitric acid solution B enters by the second falling film absorption tower absorption liquid inlet, the content of the nitrosyl sulfuric acid of the second falling film absorption tower absorption liquid is After 10-20wt%, circulate to the first falling film absorption tower, after the concentration of the absorption liquid of the first falling film absorption towe...
Embodiment 2
[0062] Compared with Example 1, the difference is that the mixed nitric acid solution B is a mixed solution of 0.7:1 nitric acid and sulfuric acid in a mass ratio. The content of sulfur dioxide is 50.06wt%, and the absorption rate of sulfur dioxide is 98.7%.
Embodiment 3
[0064] Compared with Example 1, the difference is that the mixed nitric acid solution B is a mixed solution of 0.54:1 nitric acid and sulfuric acid in a mass ratio. The content of sulfur dioxide is 58.06wt%, and the absorption rate of sulfur dioxide is 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com