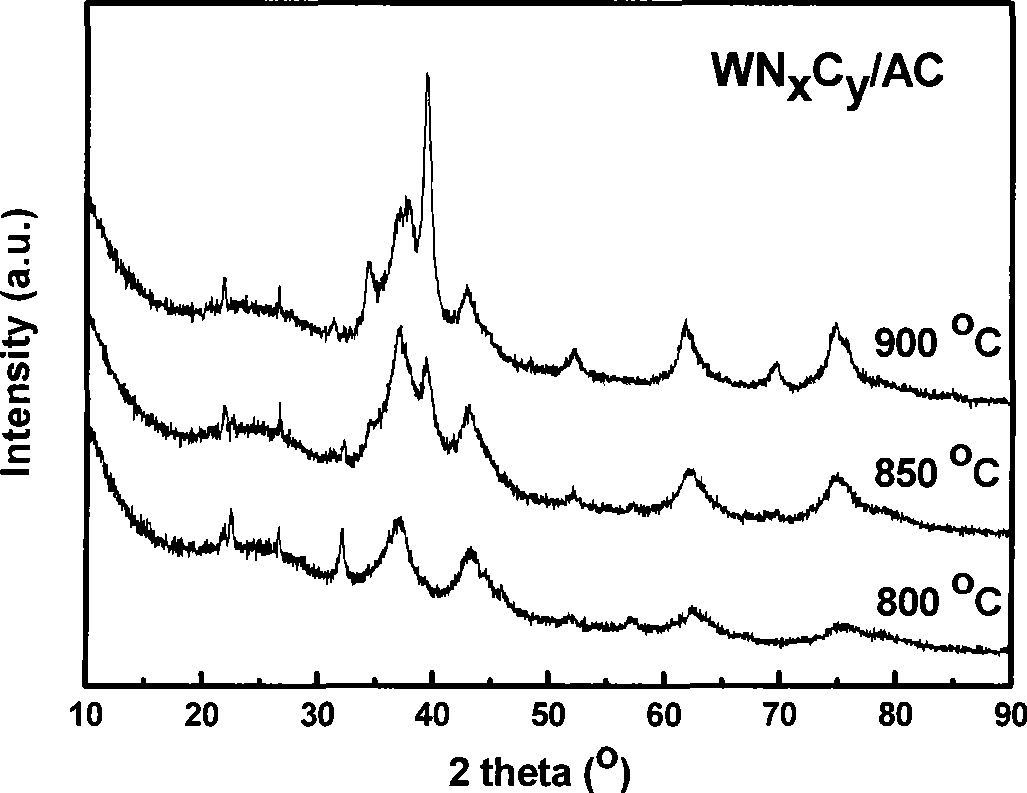
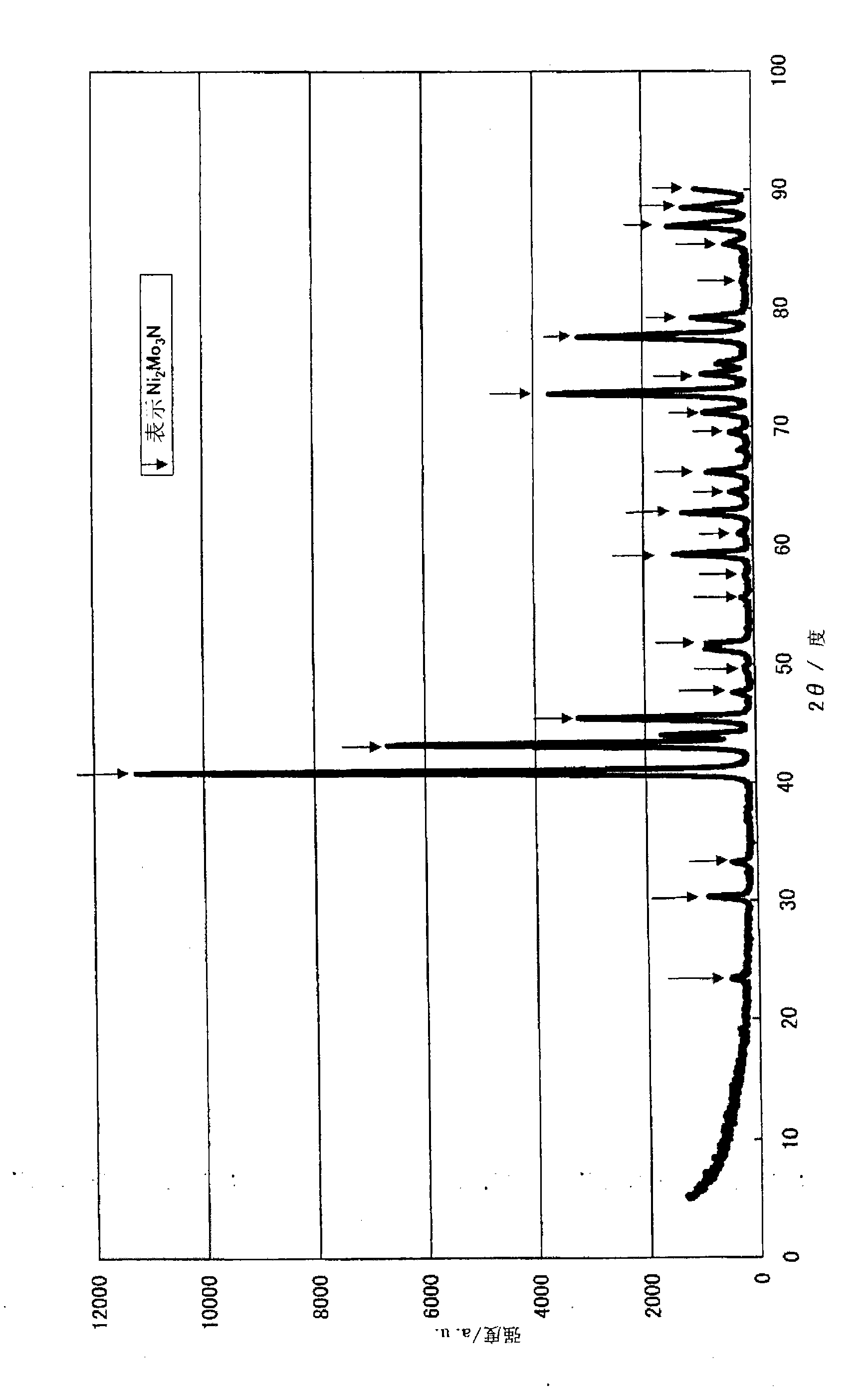
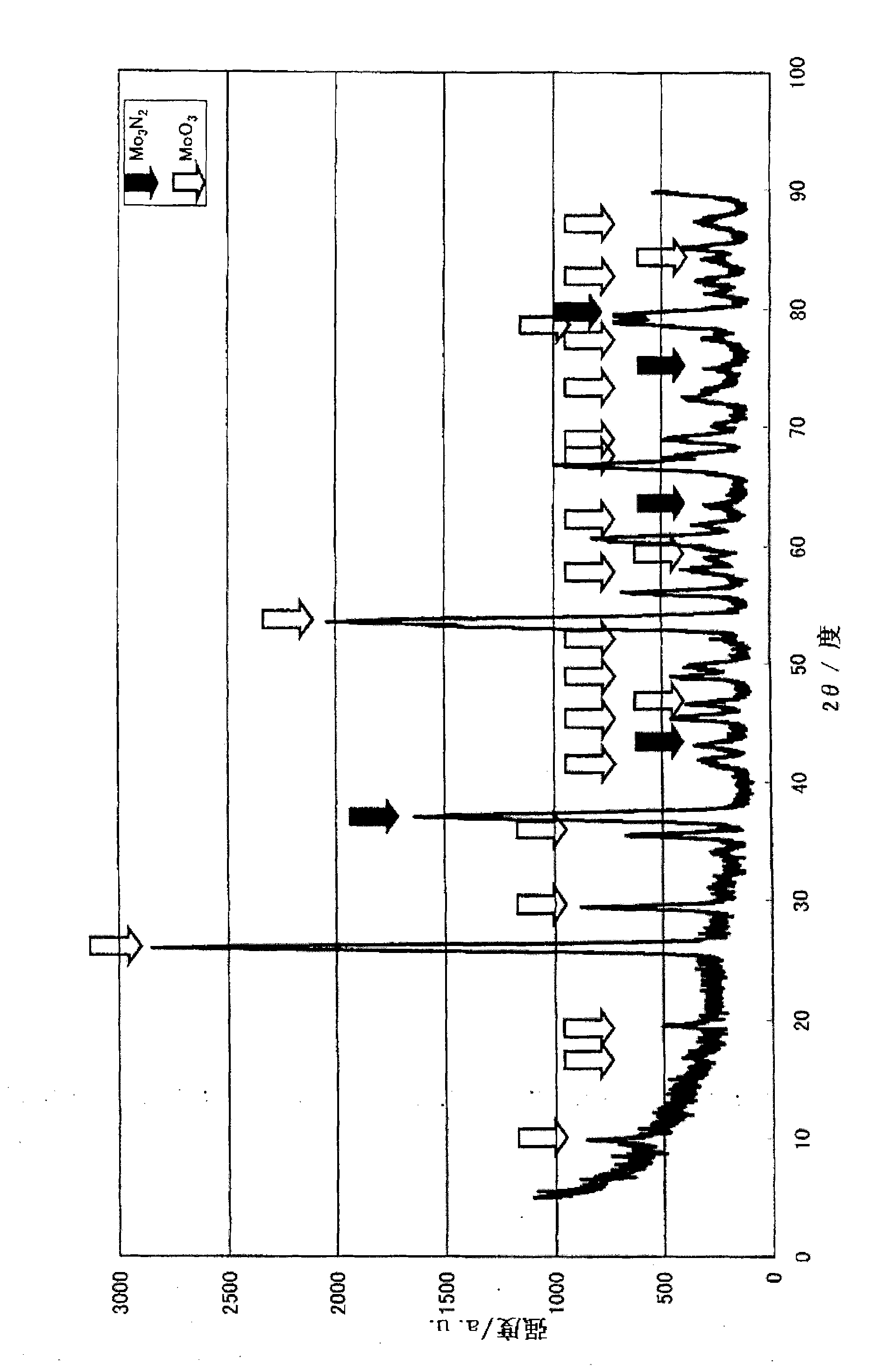
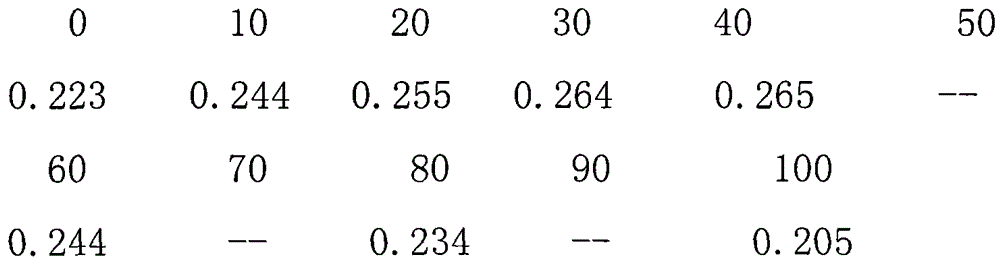Patents
Literature
194results about "Nitrogen preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
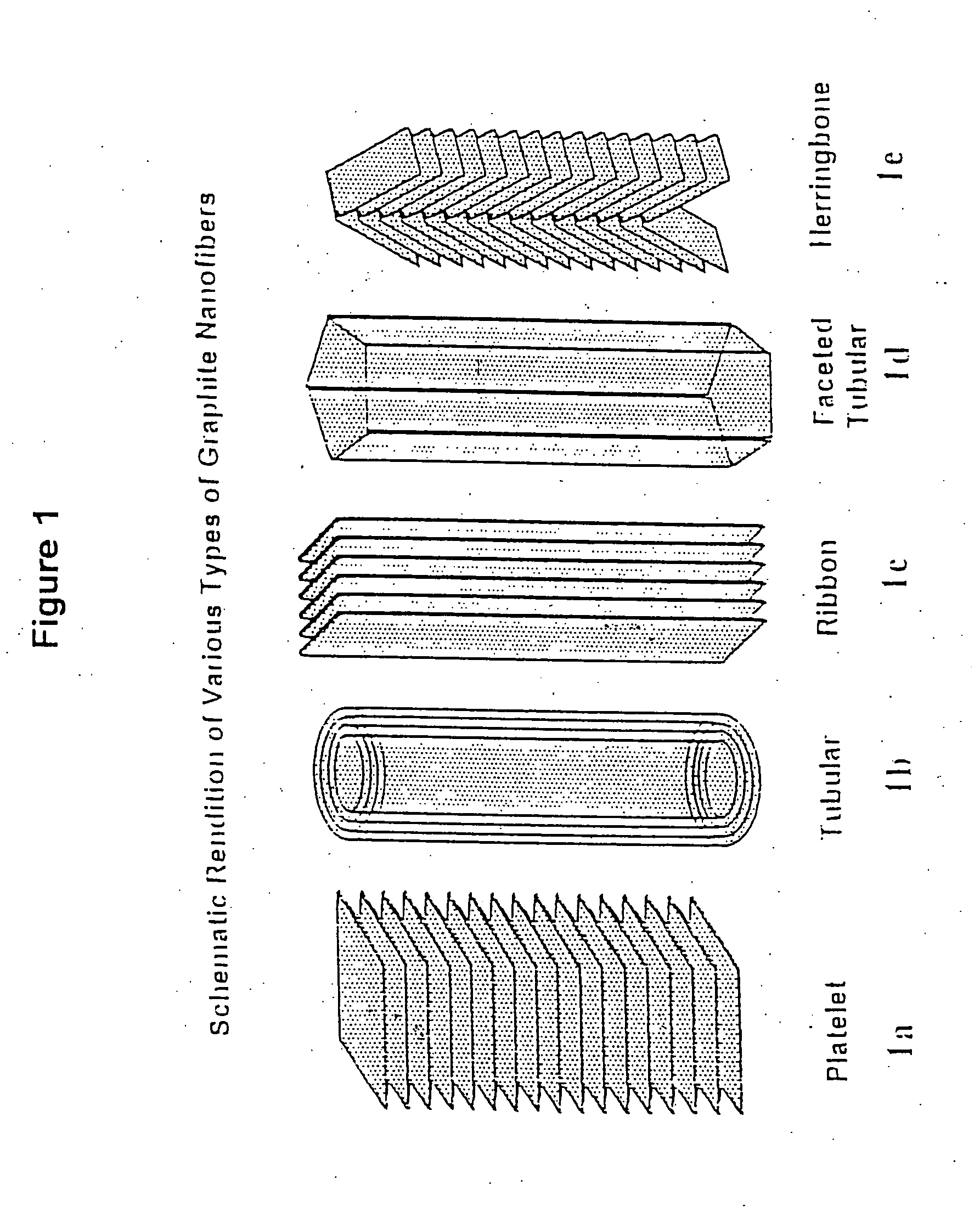
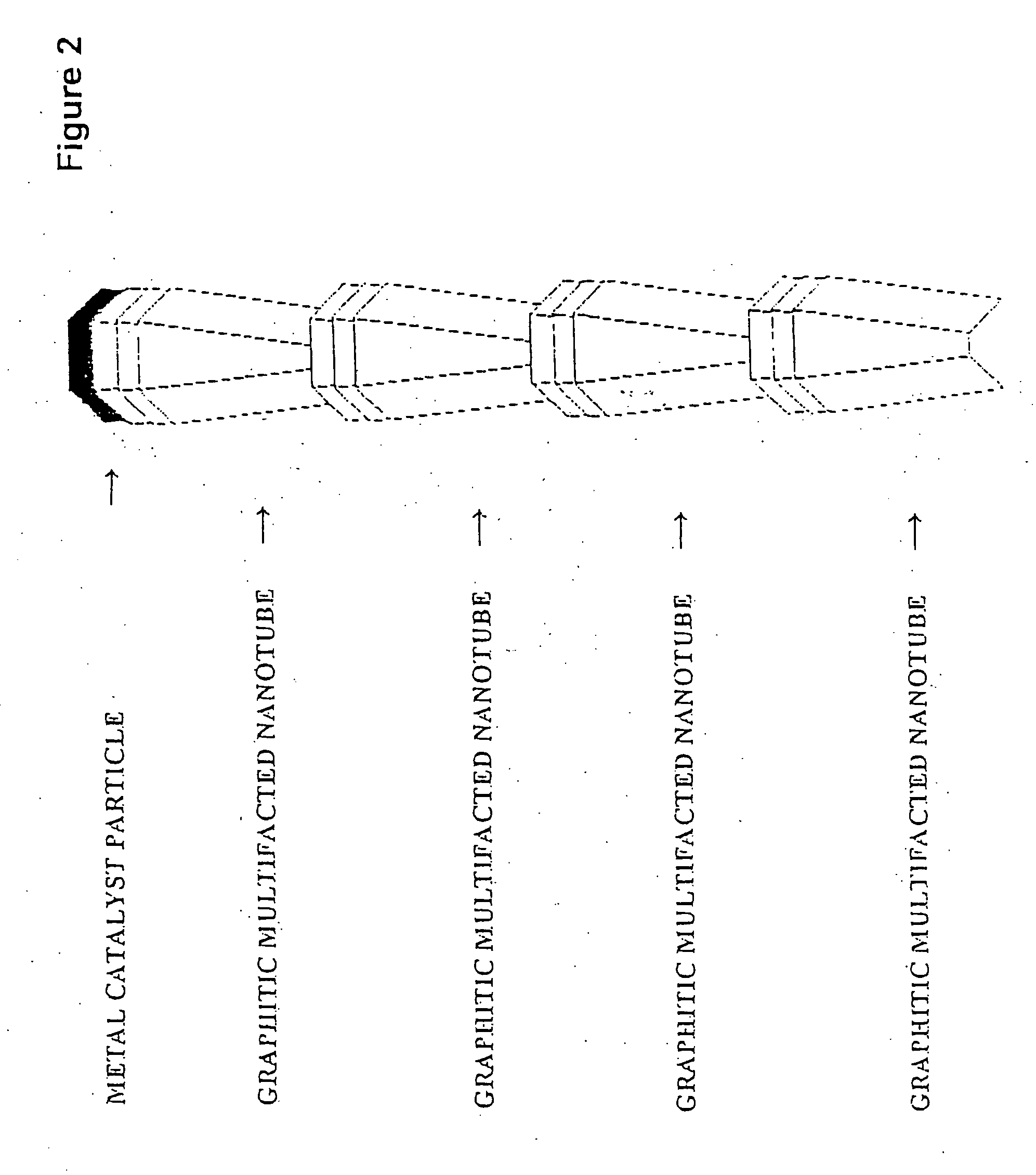
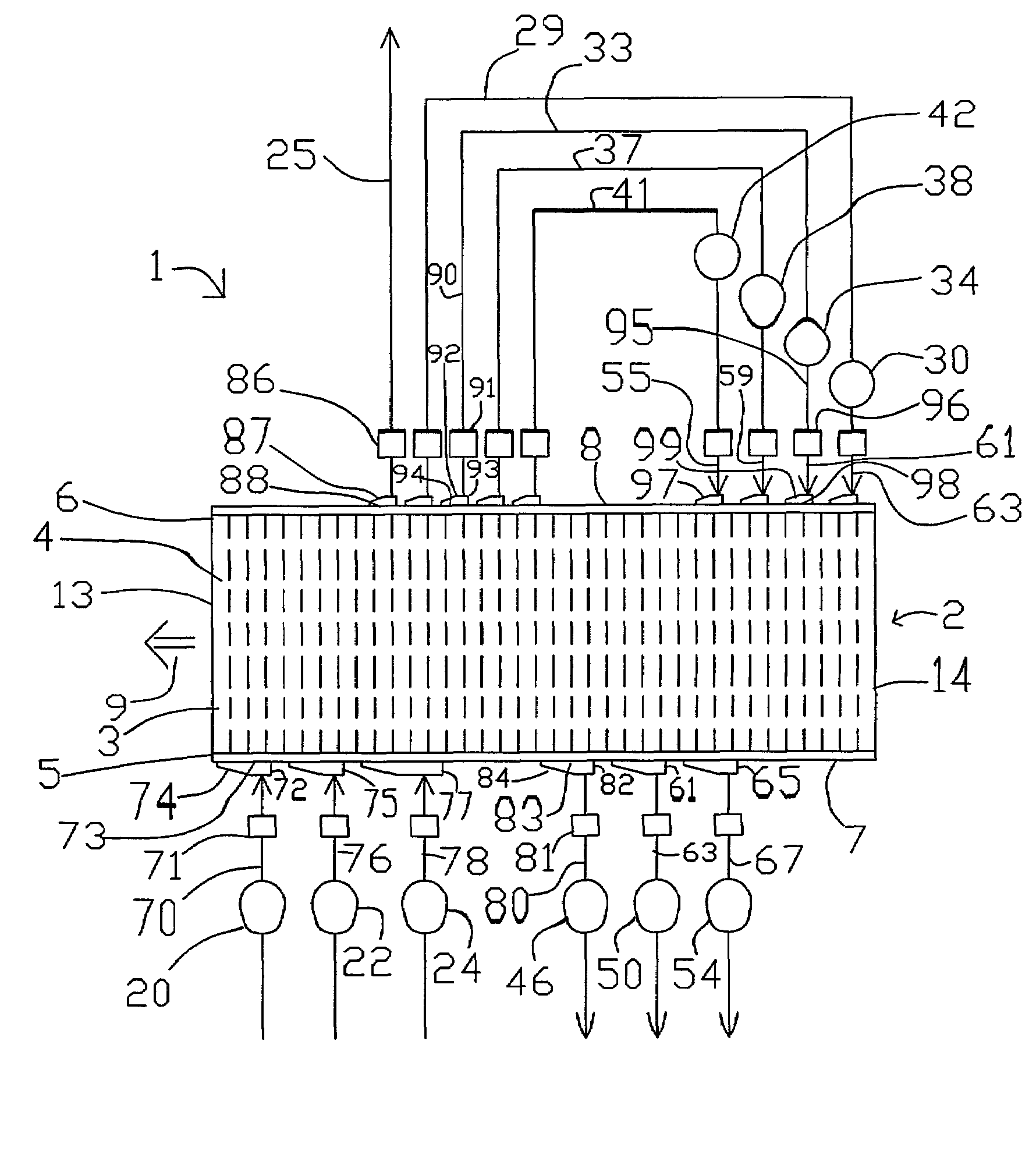
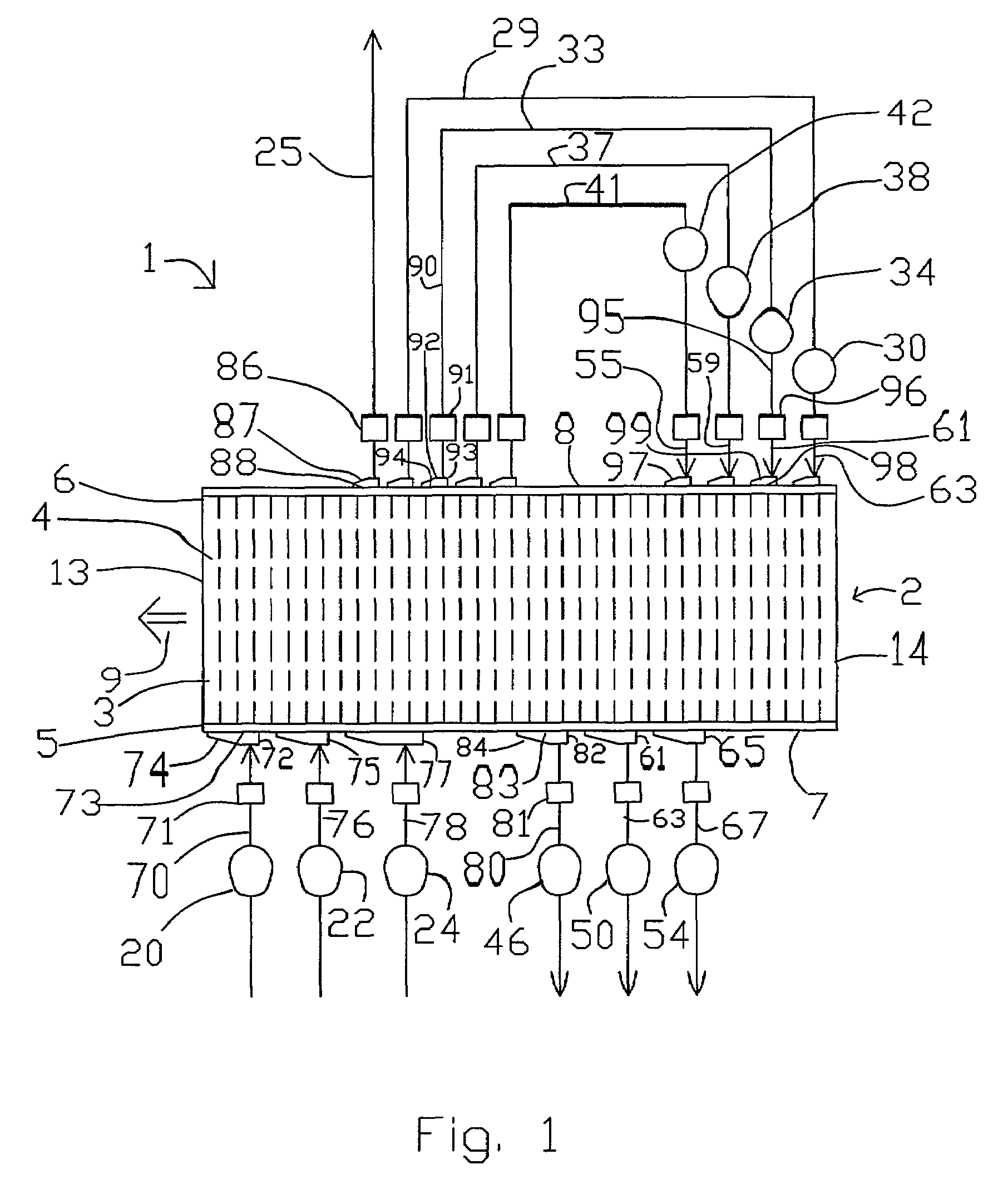
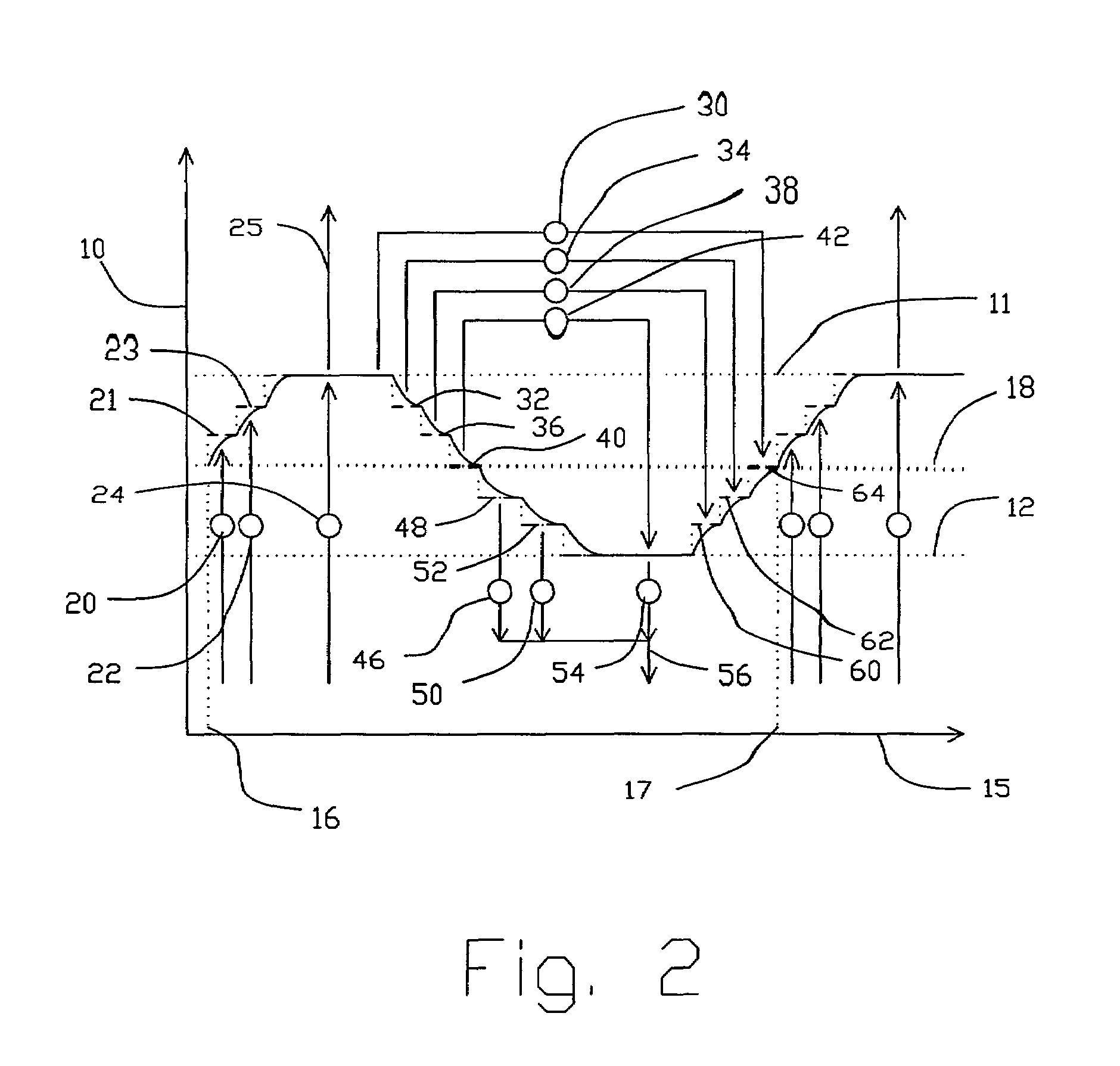
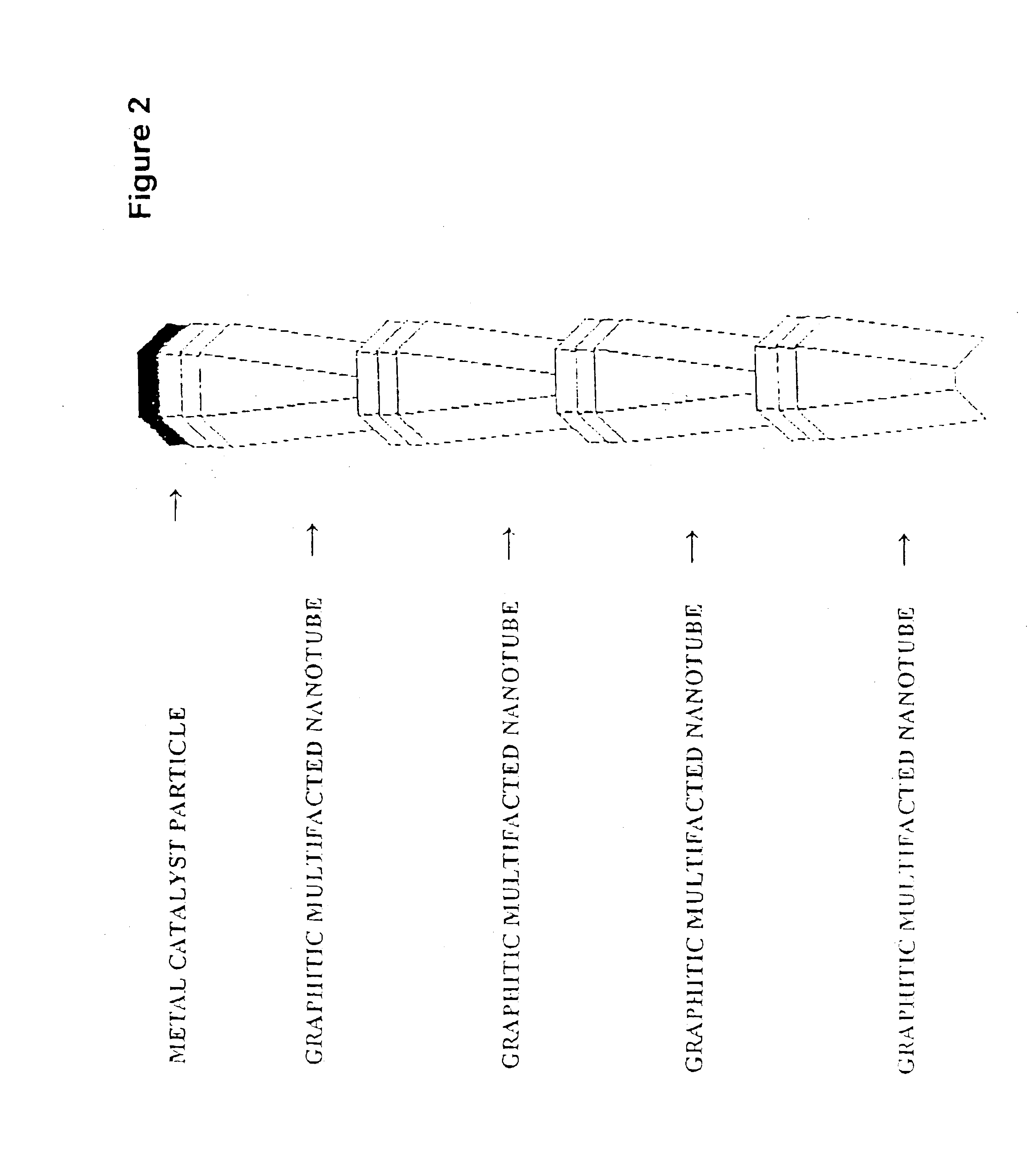
Novel graphite nanocatalysts
Novel catalysts comprised of graphitic nanostructures. The graphitic nanostructure catalysts are suitable for catalyzing reactions such as oxidation, hydrogenation, oxidative-hydrogenation, and dehydrogenation.
Owner:CATALYTIC MATERIALS
Method for preparing three-dimensional graphene-carbon nitrogen nanotube composite
InactiveCN102745679ASimple preparation processLow costMaterial nanotechnologyNitrogen preparationGrapheneChemical vapor deposition
The invention discloses a simple method of using foam nickel as a substrate to prepare a three-dimensional graphene-carbon nitrogen nanotube composite. Effective composite of three-dimensional graphene and carbon nanotubes is realized by directly growing the carbon nanotubes on the surface of the three-dimensional graphene by means of the two-step chemical vapor deposition technique, and functionalization of a carbon-based three-dimensional structure is realized by introducing nitrogen-doped atoms into the carbon nanotubes. Compared with other methods for preparing the three-dimensional graphene-carbon nitrogen nanotube composite, the method has the advantages of simplicity in preparation process, low cost, high product conductivity, large specific surface area and the like. The three-dimensional graphene-carbon nitrogen nanotube composite obtained by the method has excellent performances in terms of catalysis oxygen reduction reaction, electrochemical capacitors, electrochemical biological sensing, super-hydrophobic oleophylic foams and the like, and has high potential application values in terms of ionic cells, drug transmission, microreactors and the like.
Owner:NANJING UNIV OF POSTS & TELECOMM
Urea based composition and system for same
InactiveUS20030219371A1Safe handlingLow degreeGas turbine plantsFused electrolyte fuel cellsNitrogenNitrogen gas
A method and apparatus for generating energy from a composition comprising urea and water are described. The method in one embodiment includes: (a) reacting the urea with water to form ammonia; and (b) oxidizing the ammonia formed in step (a) to form water and nitrogen generating energy. The apparatus in one embodiment contains: (a) a first container for providing the composition; (b) a second container for reacting the urea with water to form ammonia, wherein the second container is connected to the first container by means for delivering the composition from the first container to the second container; (c) a third container for providing ammonia, wherein the third container is connected to the second container by means for delivering ammonia from the third container to the second container; and (d) a fourth container for oxidizing ammonia to form water and nitrogen generating energy, wherein the fourth container is connected to the second container by means for delivering ammonia from the second container to the fourth container. The method and apparatus are used to generate energy for use in stationary and mobile applications.
Owner:AMENDOLA STEVEN C
Layered ammonia oxidation catalyst
The invention pertains to a layered ammonia oxidation catalyst. The layered catalyst causes ammonia to be selectively oxidized in the presence of an oxidant such as air, while minimizing the formation of nitrogen oxides (NOx). The layered catalyst comprises a refractory oxide support such as gamma alumina upon which a platinum component is deposited and a vanadia component is deposited on the platinum. The catalyst is preferably disposed on a substrate such as a metal foil whose surface contains a “herringbone” pattern.
Owner:BASF CATALYSTS LLC
Process for preparing high dispersion supported type transition metal phosphide catalyst
InactiveCN101168132AIncreased dispersionReduce reunionPhysical/chemical process catalystsNitrogen preparationPhosphateActive component
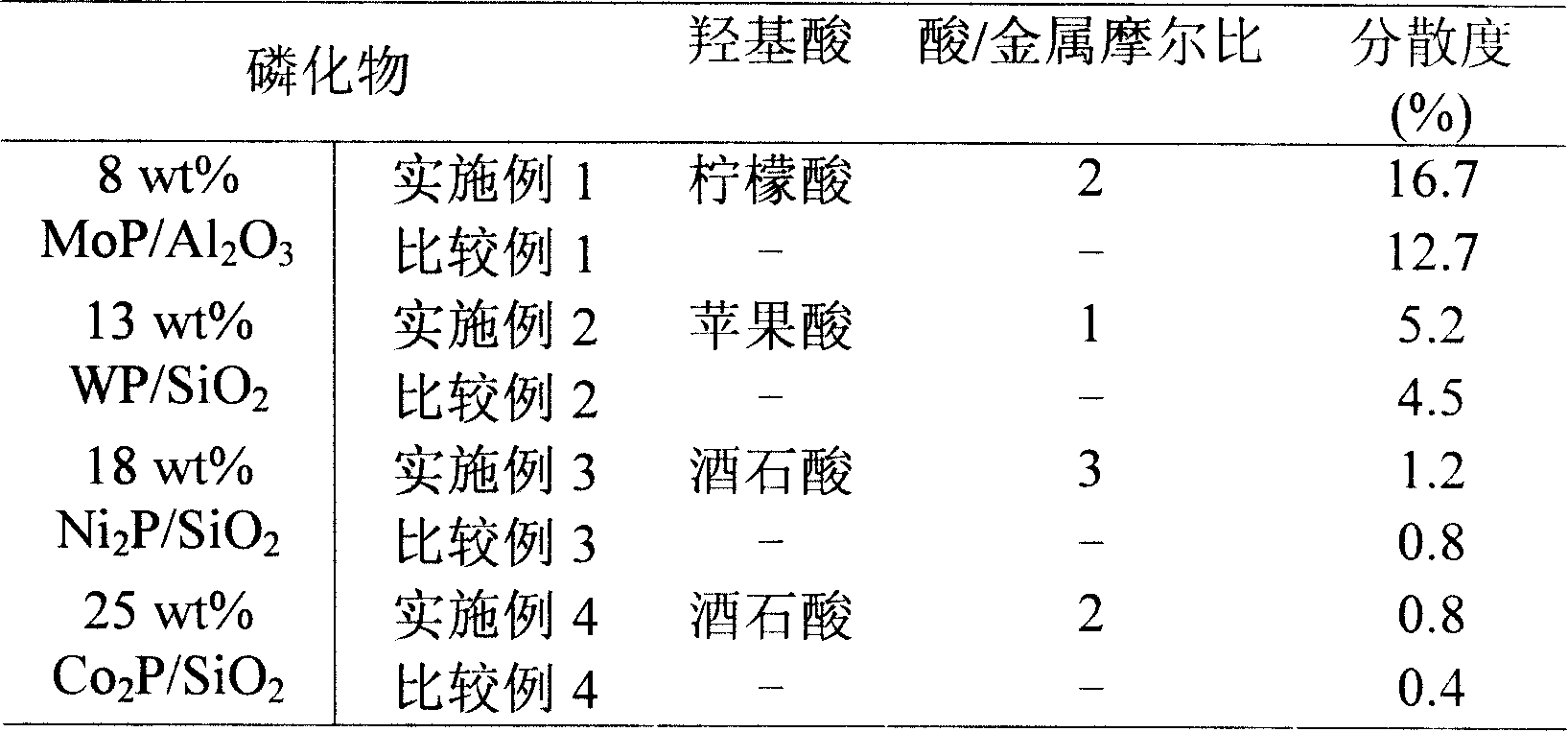
The invention relates to a catalyst of supported metal phosphide, in particular to a process for preparing the catalyst of high dispersive supported metal phosphide. The catalyst is composed of two parts of active components and a carrier which can be represented by AP / Z or B2P / Z, wherein the A of the active components is Mo or W, the B of the active components is Ni or Co, and the Z of the carrier is Al2O3 or SiO2. The operation steps of the catalyst are that transient metal salt and mammonium dihydrogen phosphate are dissolved in deionized water according to the stoichiometric ratio of targeted phosphide, and simultaneously moderate hydroxy acid as chelating agent is added to get the impregnating solution for preparing supported transition metal phosphide. The porous carrier is immersed in the impregnating solution, and is rested, the procedure gains temperature and returns after the impregnating solution is dried, roasted and under the atmosphere of hydrogen gas, and finally the supported transition metal phosphide is made. The supported transition metal phosphide prepared by the preparation method has the advantages of high dispersion, simple and convenient operation, low cost, no pollution, good repeatability and easiness of large scale preparation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method and devices for heating urea-containing materials in vehicle emission control system
InactiveUS20120045378A1Reduce nitrogen oxide emissionsEfficient productionPressurized chemical processInternal combustion piston enginesThermal energyThermal energy storage
Owner:DOW GLOBAL TECH LLC
Process for the treatment of waste streams
The present invention is directed to a process for treating, reducing, and / or stabilizing various wastes or flue gases. In one embodiment, the process is directed to treatment of alkali bearing wastes that include nitrate and / or nitrite-rich wastes. Optionally, the disclosed method can be utilized for treatment of hazardous wastes, including radioactive hazardous waste compounds. In general, the present invention includes processing a waste or gaseous stream with the addition of suitable carbon-containing additives to treat and reduce nitrogen-containing compounds in the waste. Additives may be gaseous, liquid or solid reduction-promoting agents, catalysts, and the like. The reaction products obtained from the process of the invention include mainly alkali carbonate, nitrogen, hydrogen, carbon monoxide and carbon dioxide.
Owner:MFG & TECH CONVERSION INT
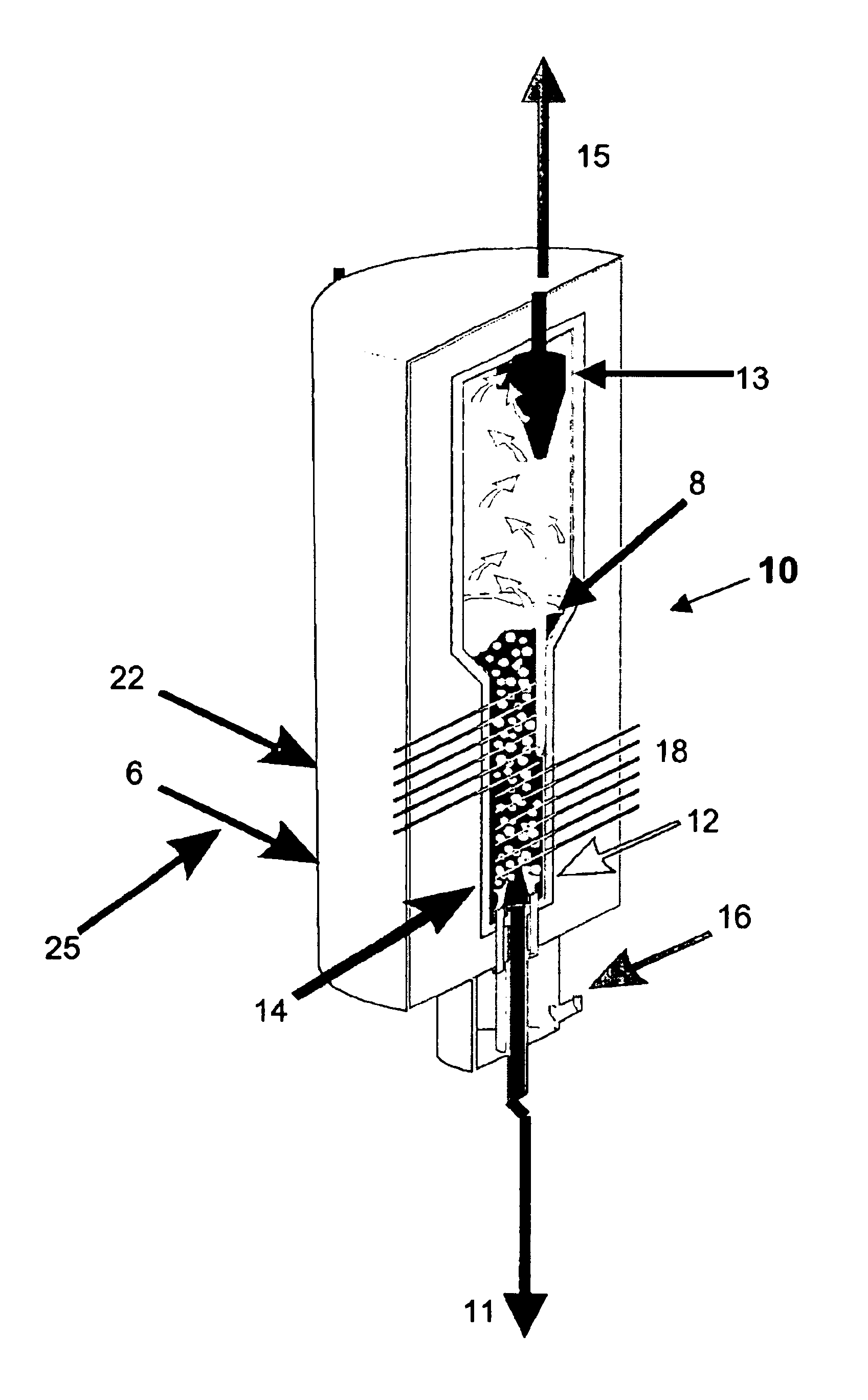
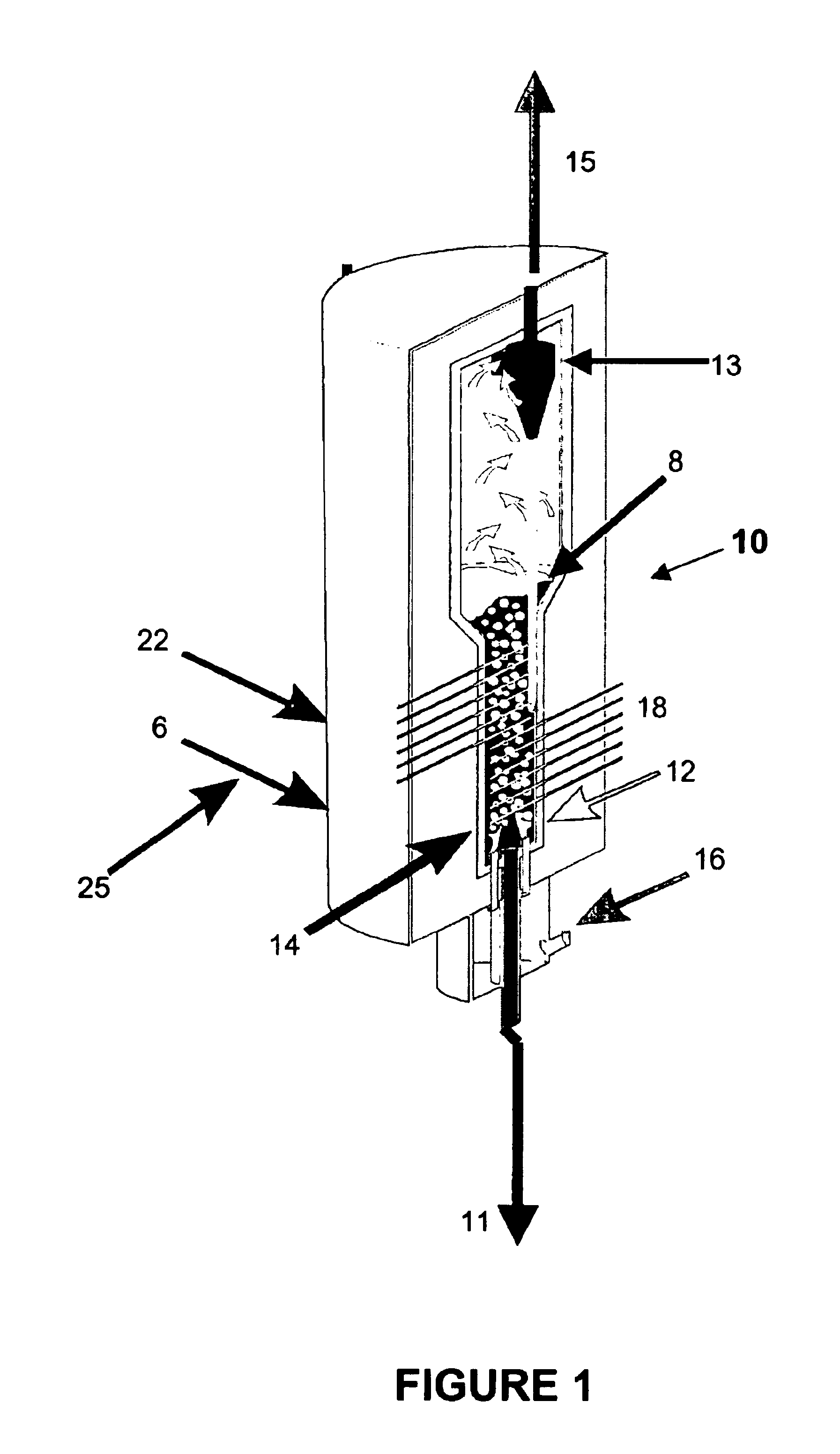
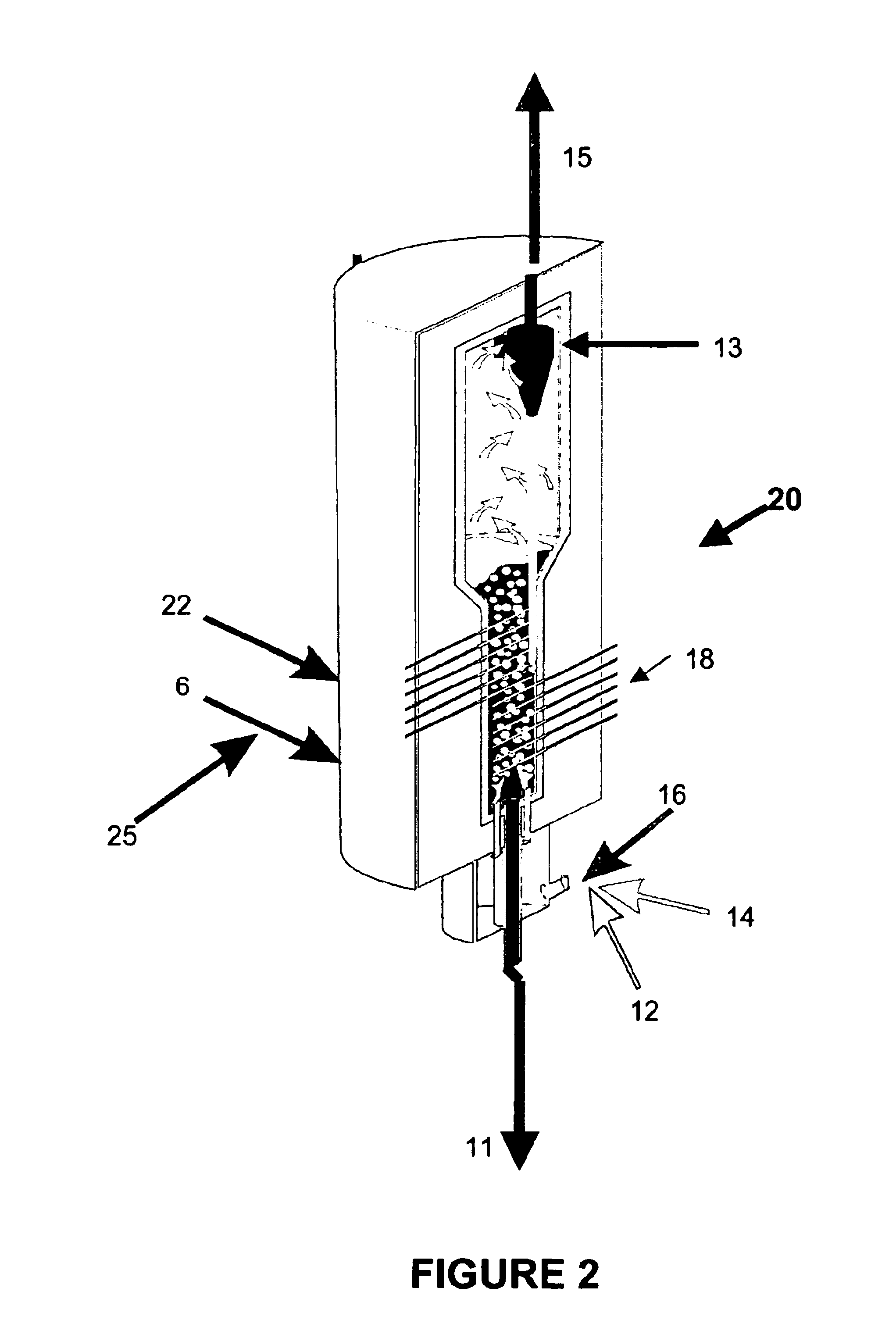
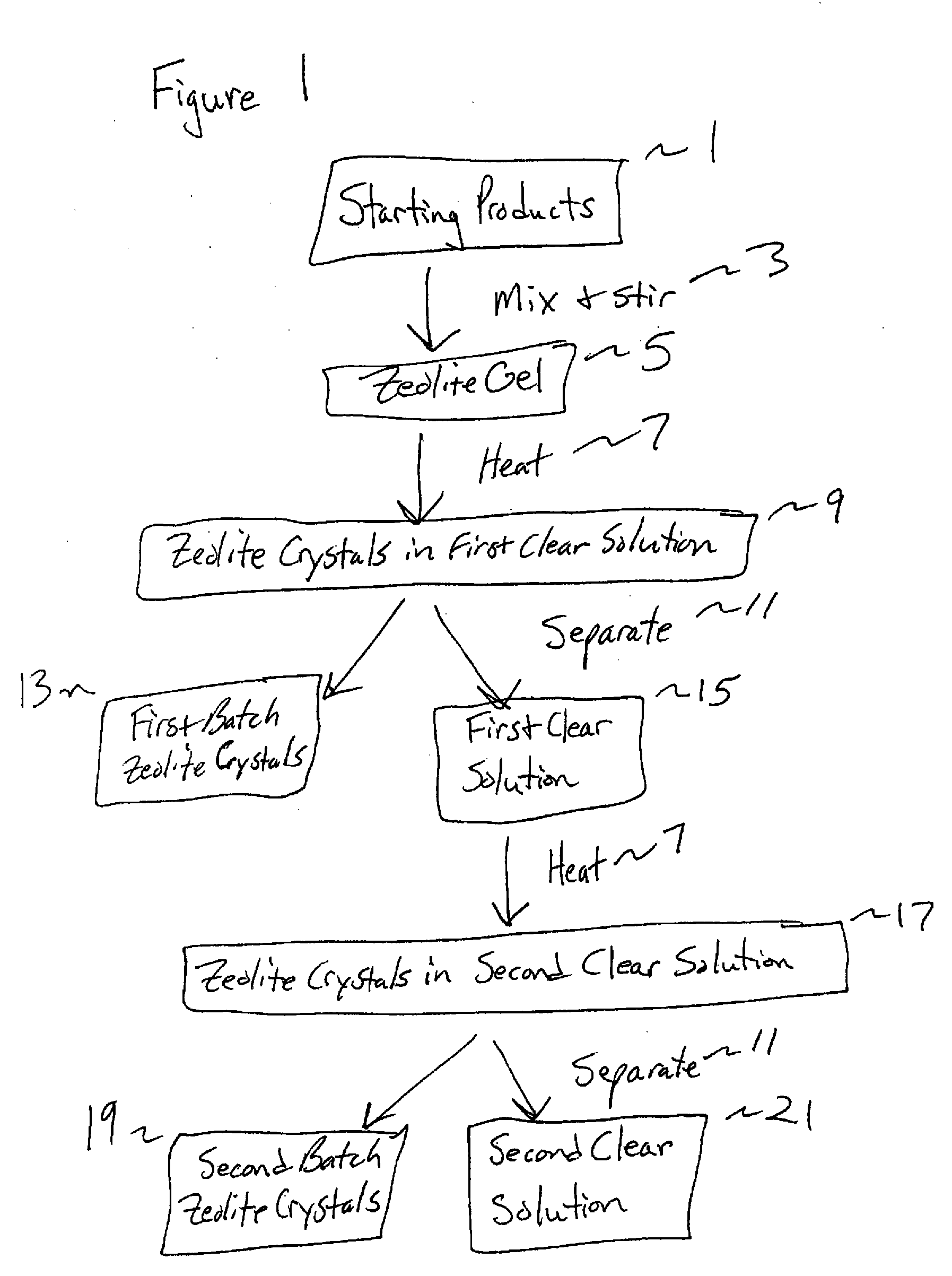
Synthesis and use of nanocrystalline zeolites
InactiveUS20070071666A1High yieldMore consistent crystal sizeNitrous oxide captureMaterial nanotechnologySolventHydrolysis
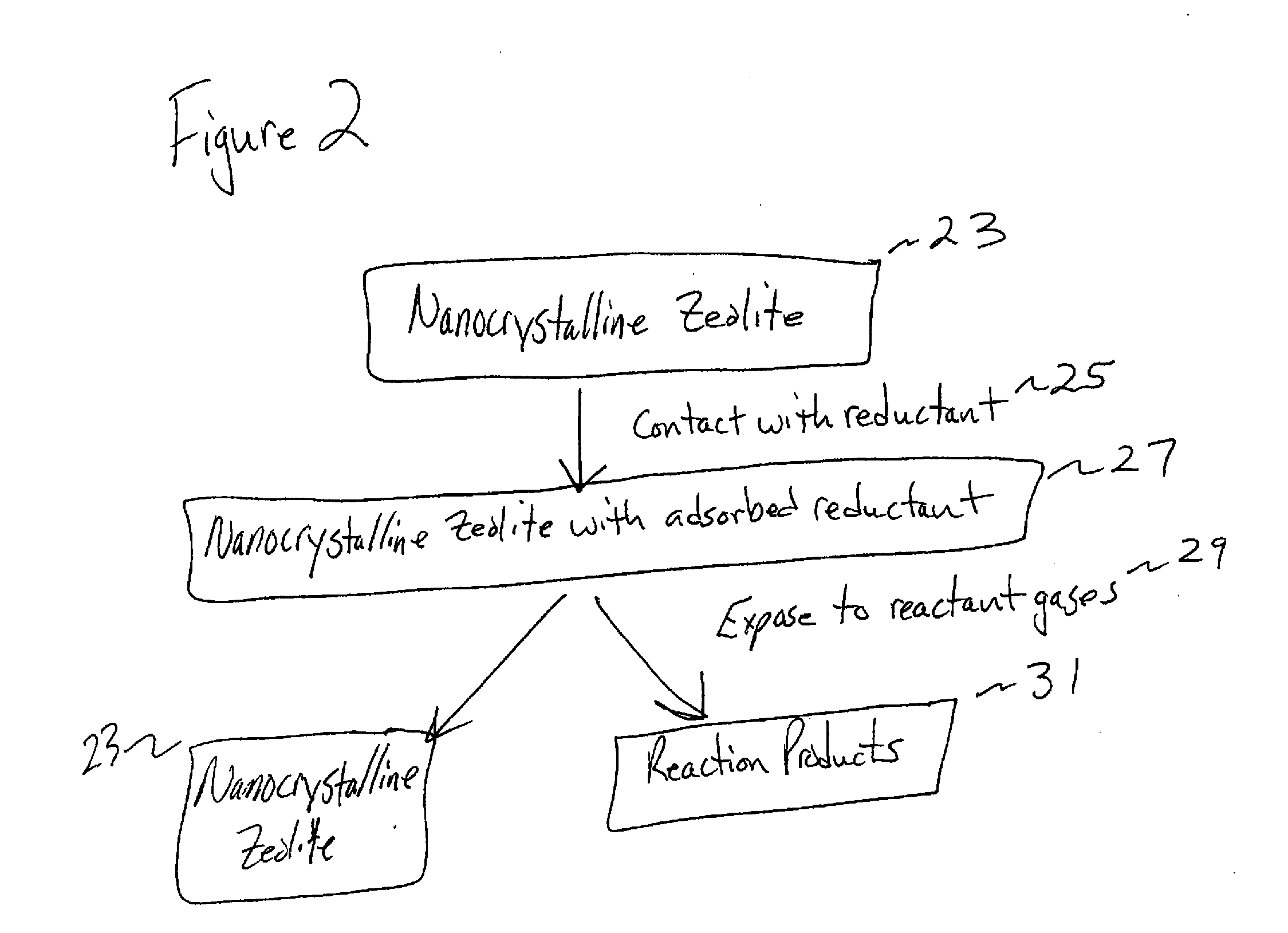
Embodiments of the present invention relate to a method for synthesizing nanocrystalline zeolites, the method comprising contacting starting products that comprise a solvent, a silicon source, a cation base, an organic template, and an aluminum source, or any combination thereof sufficient to produce a zeolite gel by hydrolysis, heating the zeolite gel sufficient to produce a first batch of zeolite crystals and a first clear solution, separating the first batch of zeolite crystals from the first clear solution, heating the first clear solution sufficient to produce a second batch of zeolite crystals and second clear solution and separating the second batch of zeolite crystals from the second clear solution. In addition, embodiments relate to a method of using nanocrystalline zeolites, the method comprising contacting a nanocrystalline zeolite with a reductant sufficient to produce a nanocrystalline zeolite with adsorbed reductant and exposing the nanocrystalline zeolite with adsorbed reductant to reactant gases sufficient to obtain reaction products and the nanocrystalline zeolite.
Owner:UNIV OF IOWA RES FOUND
Ammonia decomposition catalysts and their production processes, as well as ammonia treatment method
InactiveUS20110176988A1Efficient decompositionSimple and easy mannerGas treatmentNitrogen preparationDecompositionIron group
The ammonia decomposition catalyst of the present invention is a catalyst for decomposing ammonia into nitrogen and hydrogen, including a catalytically active component containing at least one kind of transition metal selected from the group consisting of molybdenum, tungsten, vanadium, chromium, manganese, iron, cobalt, and nickel, preferably including: (I) a catalytically active component containing: at least one kind selected from the group consisting of molybdenum, tungsten, and vanadium; (II) a catalytically active component containing a nitride of at least one kind of transition metal selected from the group consisting of molybdenum, tungsten, vanadium, chromium, manganese, iron, cobalt, and nickel; or (III) a catalytically active component containing at least one kind of iron group metal selected from the group consisting of iron, cobalt, and nickel, and at least one metal oxide, thereby making it possible to effectively decompose ammonia into nitrogen and hydrogen at relatively low temperatures and at high space velocities to obtain high-pure hydrogen.
Owner:NIPPON SHOKUBAI CO LTD
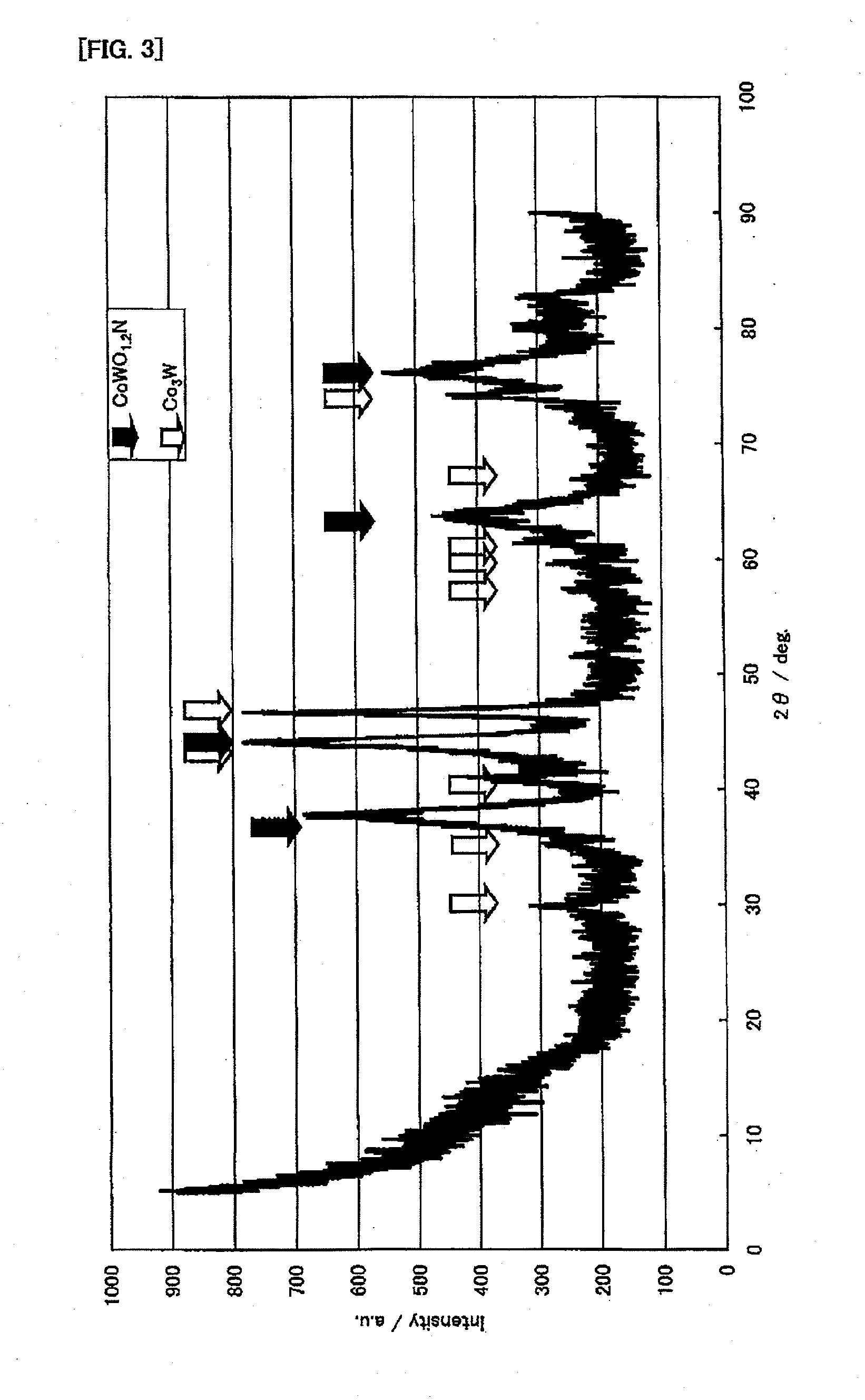
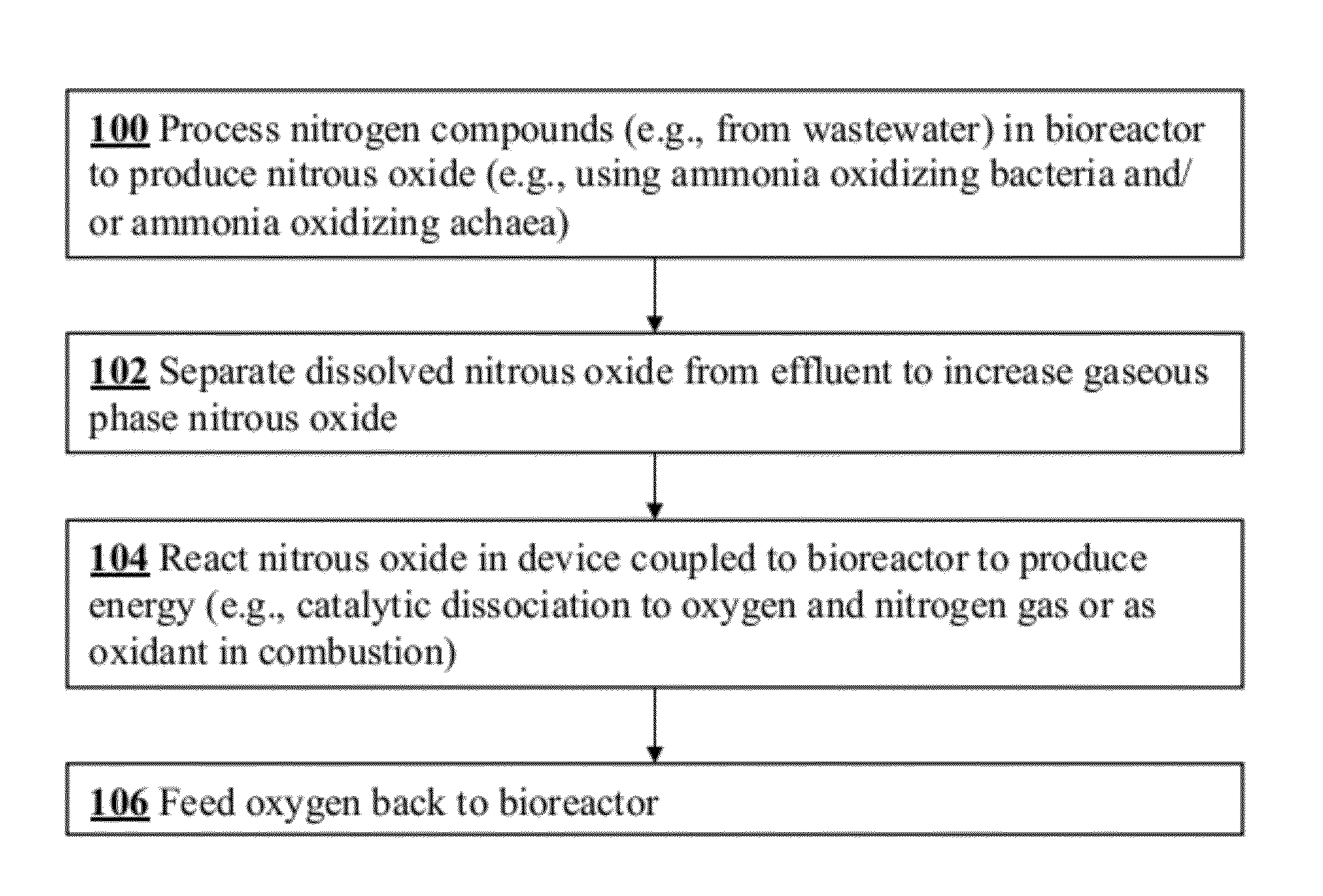
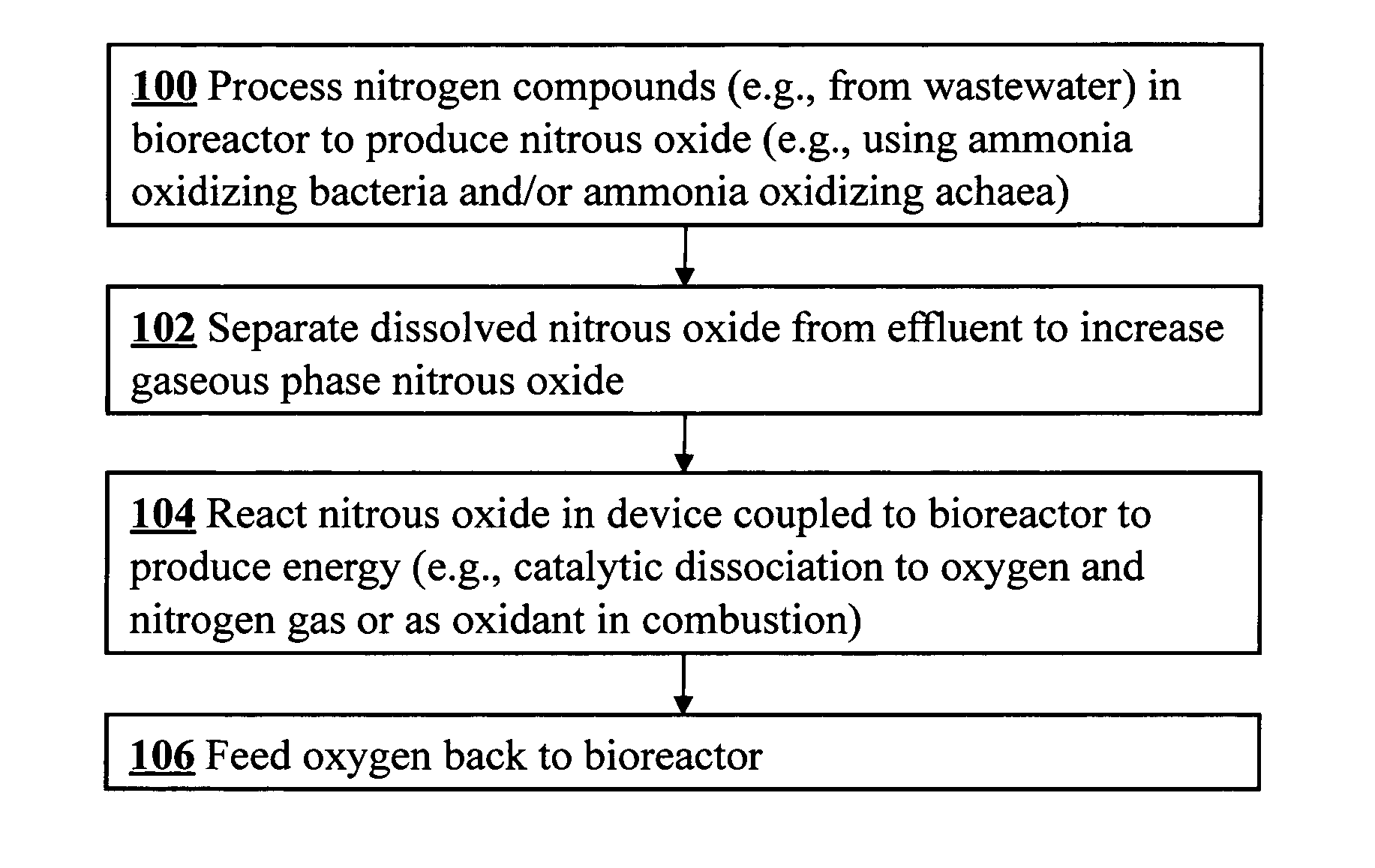
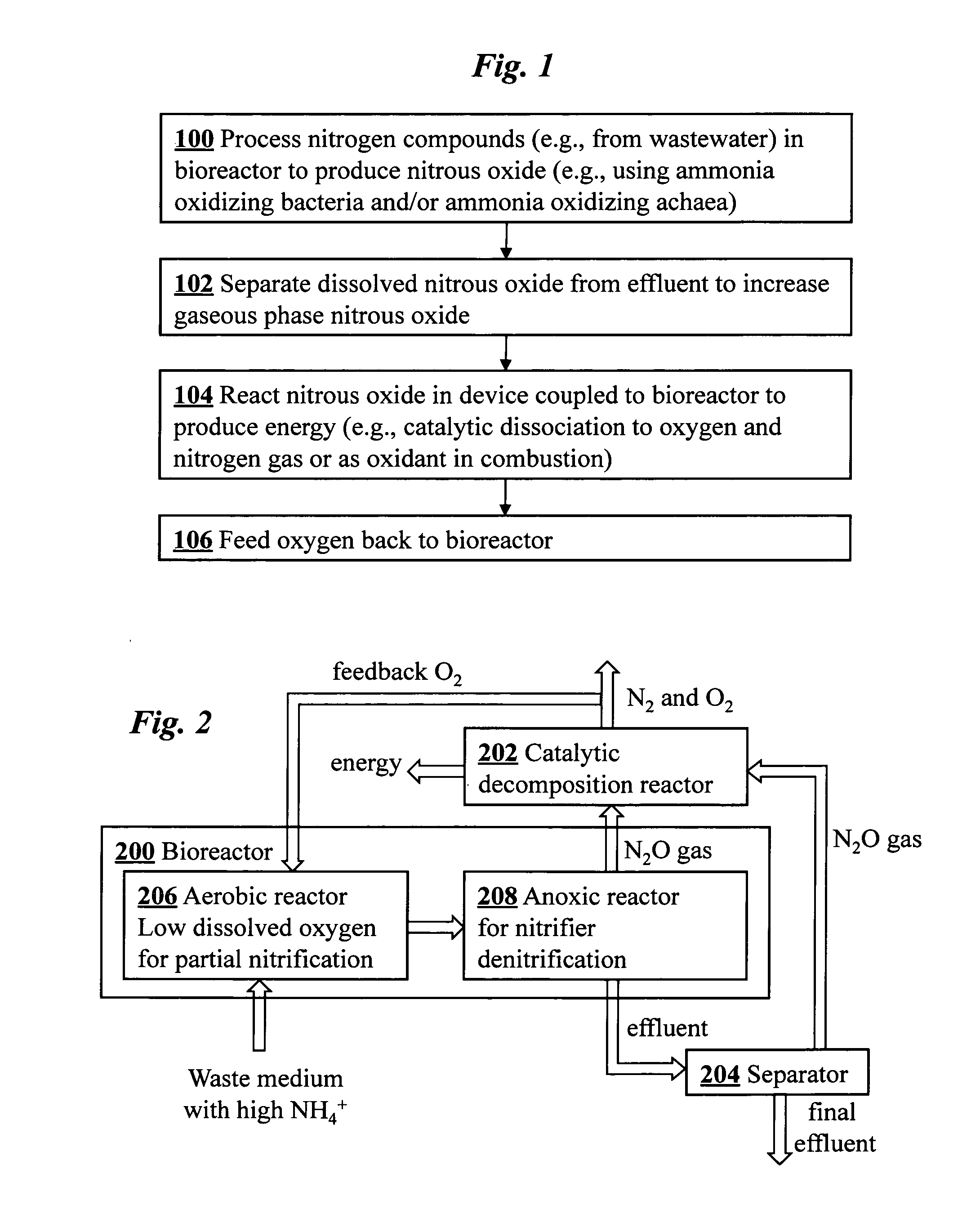
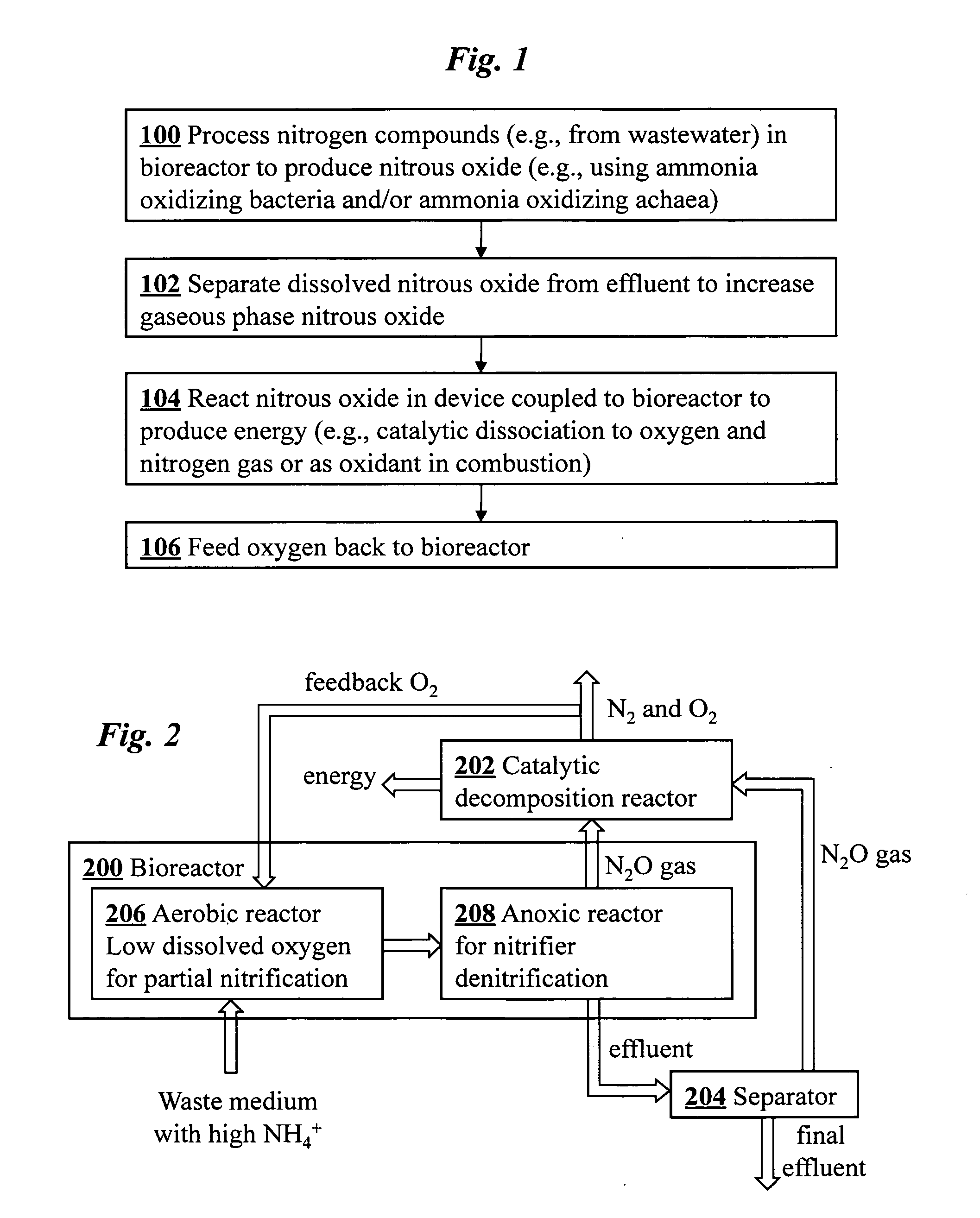
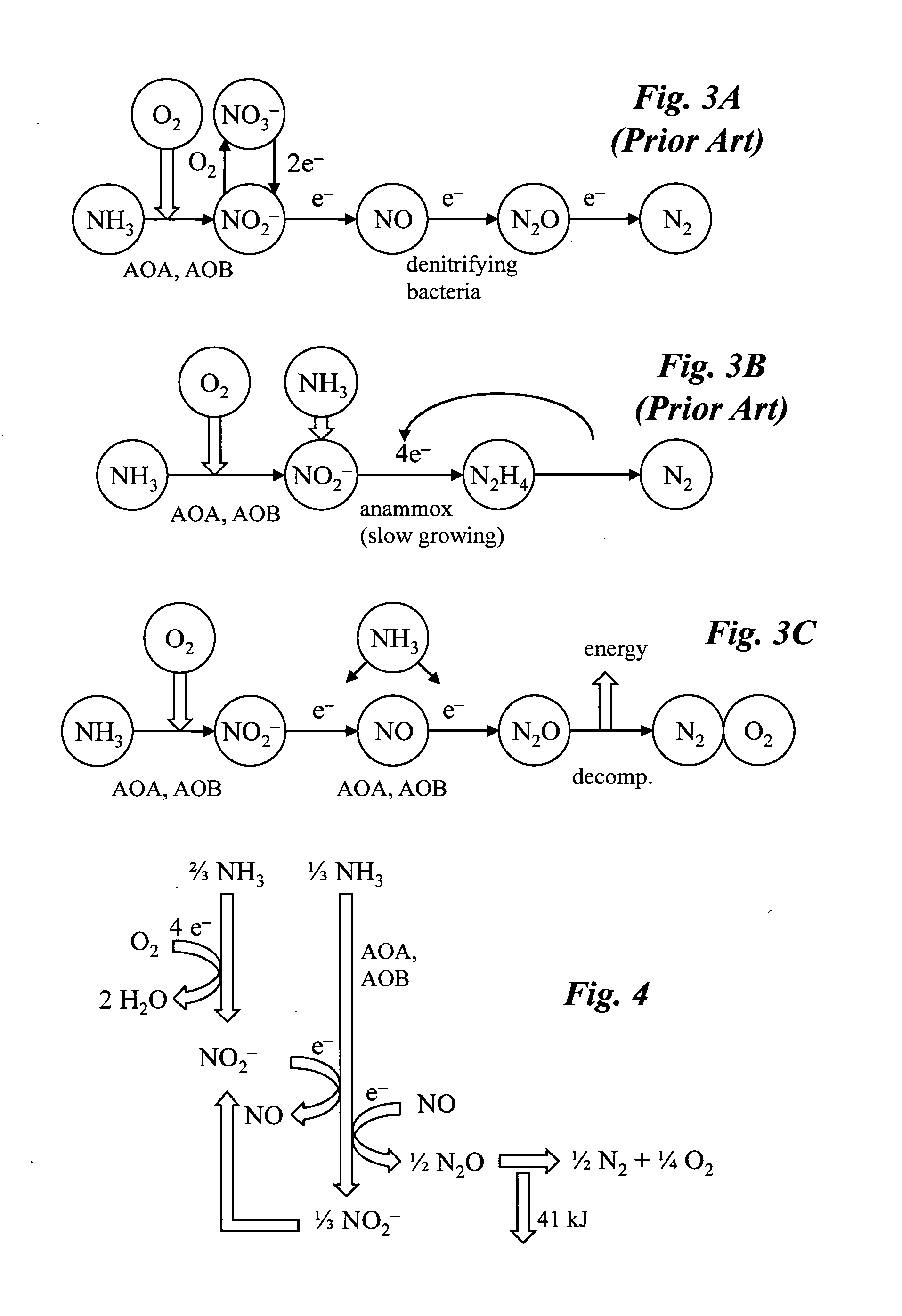
Microbial production of nitrous oxide coupled with chemical reaction of gaseous nitrous oxide including phosphorus recovery and nitrite reduction to nitrous oxide
InactiveUS20120309071A1Minimize N2O productionProducer of clean energyWater treatment parameter controlOxygen/ozone/oxide/hydroxideChemical reactionGas phase
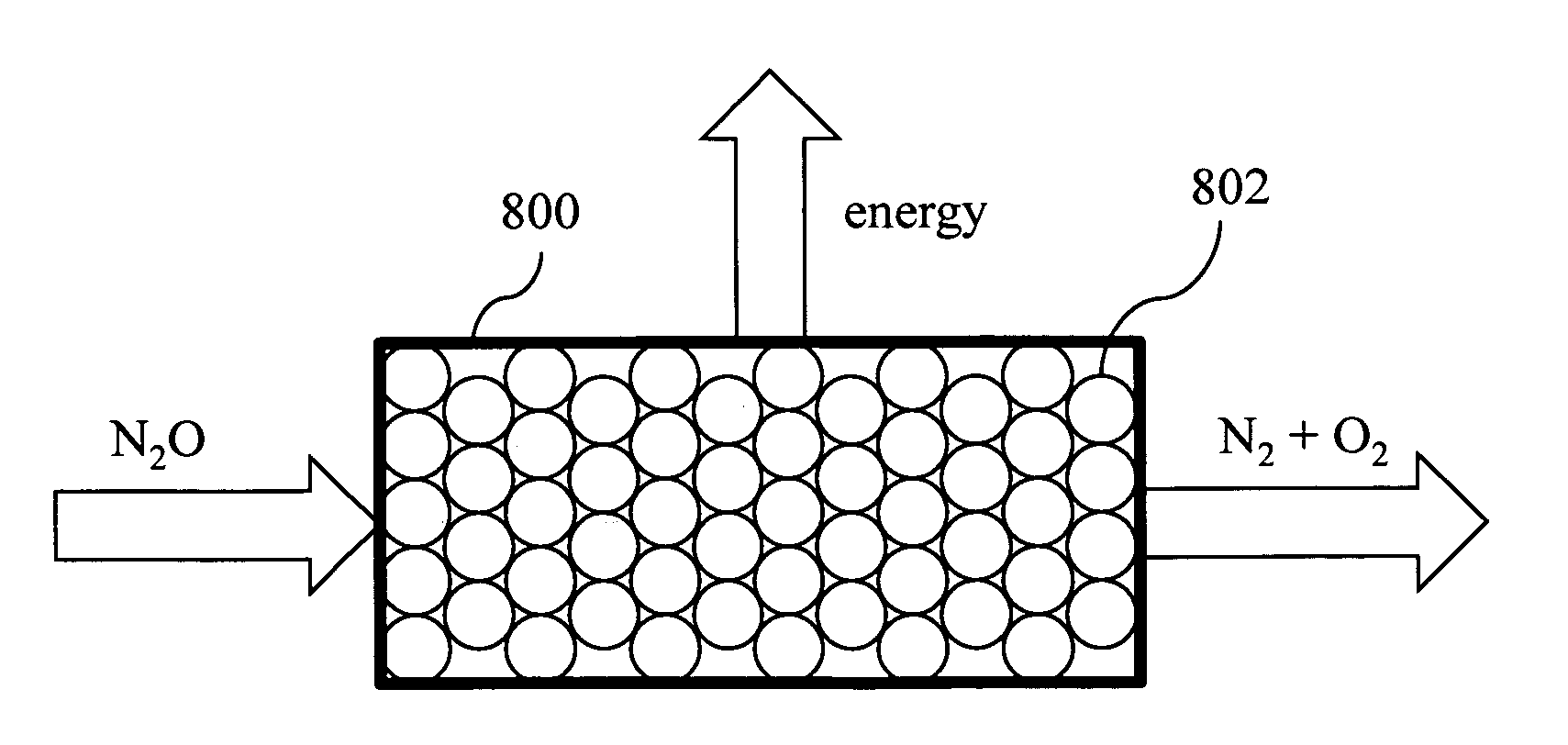
A method to produce N2O from organic nitrogen and / or reactive nitrogen in waste uses a bioreactor coupled to a hardware reactor device in which the N2O is consumed in a gas phase chemical reaction, e.g., catalytic decomposition to form oxygen and nitrogen gas. Heat from the exothermic reaction may be used to generate power. The N2O may alternatively be used as an oxidant or co-oxidant in a combustion reaction, e.g., in the combustion of methane.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Chemical reactor with pressure swing adsorption
InactiveUS7250150B1Improve energy efficiencyCompact machineryOrganic chemistry methodsHydroxy compound preparationChemical reactionChemical reactor
A chemical reaction is performed with separation of the product(s) and reactant(s) by pressure swing adsorption (PSA), using an apparatus having a plurality of adsorbers cooperating with first and second valve assemblies in a PSA module. The PSA cycle is characterized by multiple intermediate pressure levels between higher and lower pressure of the PSA cycle. Gas flows enter or exit the PSA module at the intermediate pressure levels as well as the higher and lower pressure levels, entering from compressor stage(s) or exiting into exhauster or expander stages, under substantially steady conditions of flow and pressure. The PSA module comprises a rotor containing the adsorbers and rotating within a stator, with ported valve faces between the rotor and stator to control the timing of the flows entering or exiting the adsorbers in the rotor. The reaction may be performed within a portion of the rotor containing a catalyst.
Owner:AIR PROD & CHEM INC
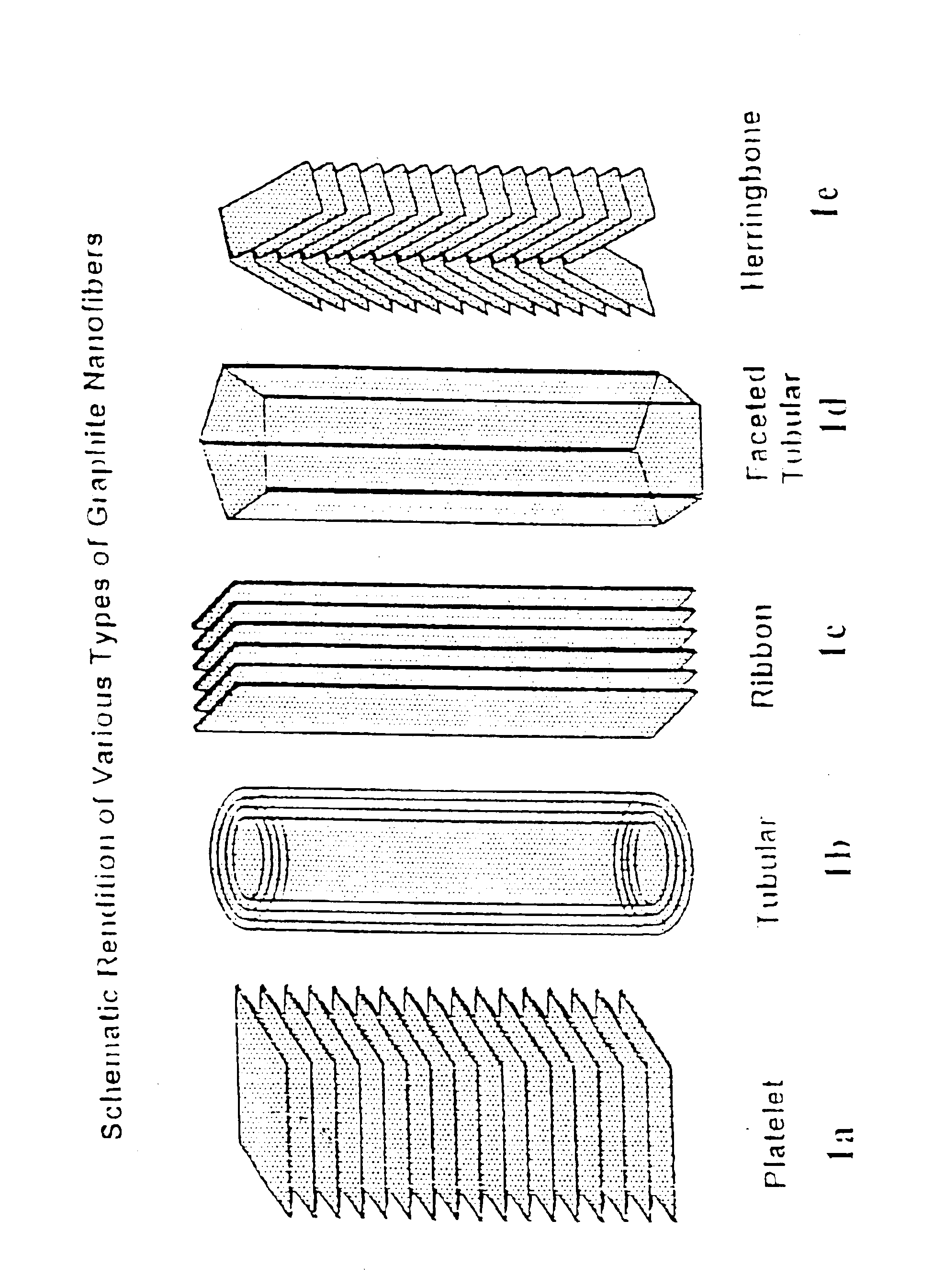
Graphite nanocatalysts
Novel catalysts comprised of graphitic nanostructures. The graphitic nanostructure catalysts are suitable for catalyzing reactions such as oxidation, hydrogenation, oxidative-hydrogenation, and dehydrogenation.
Owner:CATALYTIC MATERIALS
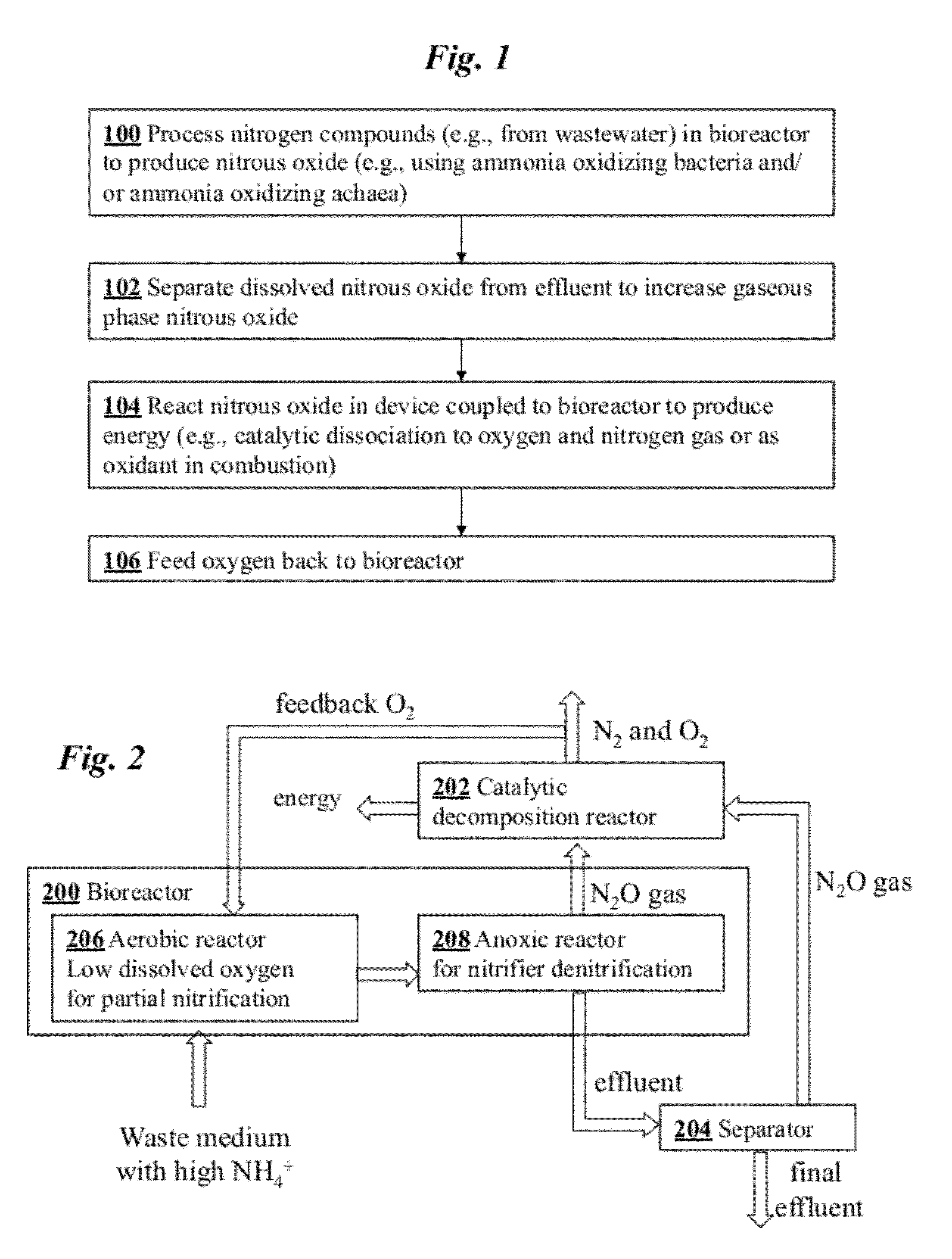
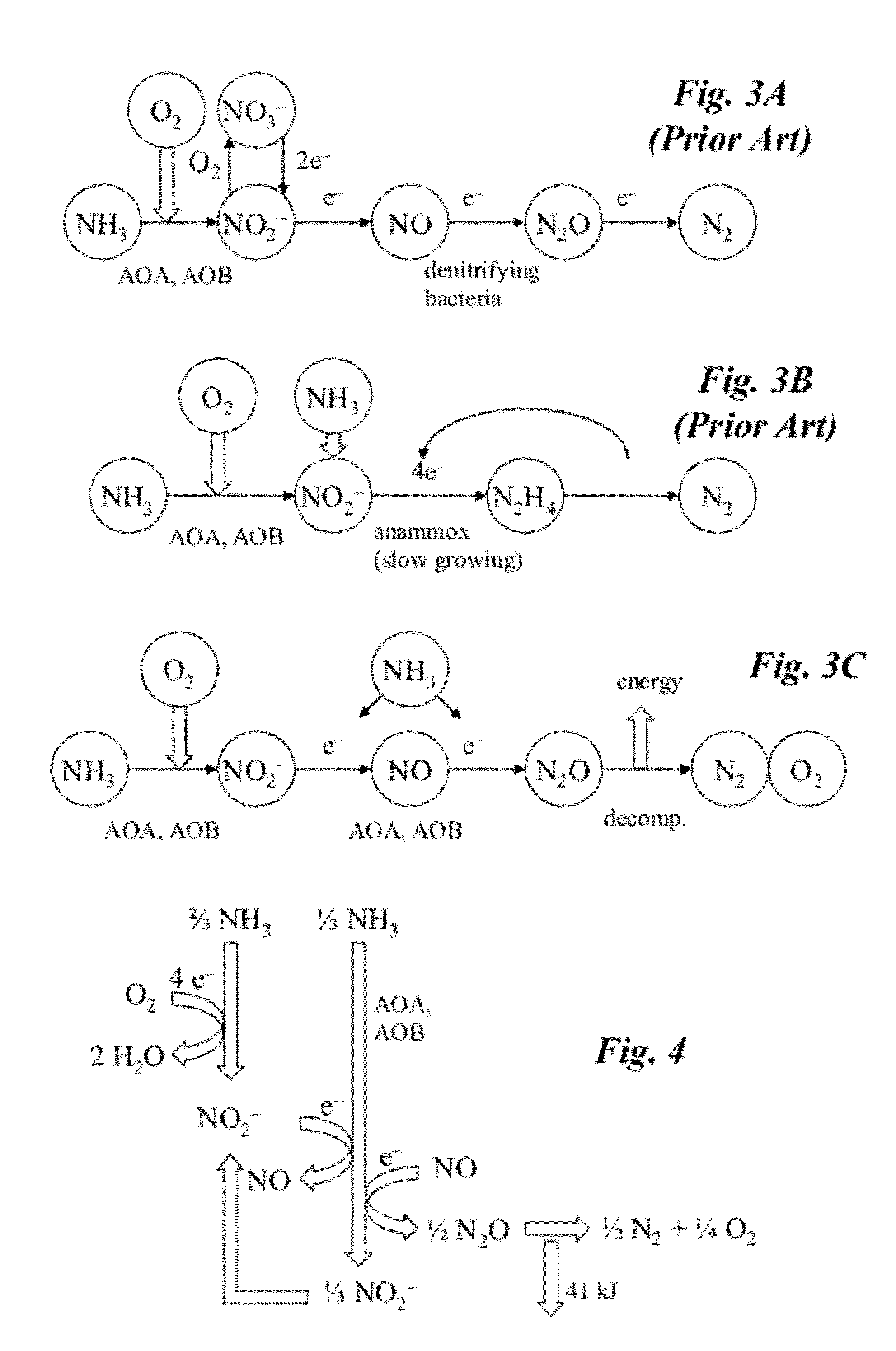
Microbial production of nitrous oxide coupled with chemical reaction of gaseous nitrous oxide
InactiveUS20110207061A1Treatment with aerobic and anaerobic processesSustainable biological treatmentSequencing batch reactorFiber
A bioreactor designed to produce N2O from organic nitrogen and / or reactive nitrogen in waste is coupled to a hardware reactor device in which the N2O is consumed in a gas phase chemical reaction, e.g., catalytic decomposition to form oxygen and nitrogen gas.Heat from the exothermic reaction may be used to generate power. The N2O may alternatively be used as an oxidant or co-oxidant in a combustion reaction, e.g., in the combustion of methane. The bioreactor may have various designs including a two-stage bioreactor, a hollow-fiber membrane bioreactor, or a sequencing batch reactor. The bioreactor may involve Fe(II)-mediated reduction of nitrite to nitrous oxide.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Low-temperature ammonia selective oxidation catalyst
ActiveCN101554587ARaw materials are readily availableEasy to prepareNitrogen preparationDispersed particle separationCeriumNitrogen gas
The invention provides a low-temperature ammonia selective oxidation catalyst which is used for eliminating ammonia gas pollution generated in the field of industry, agriculture, traffic, architecture, and the like, and selectively catalyzing and oxidizing the ammonia into pollution-free nitrogen and water under lower temperature. The oxidation catalyst can completely convert the ammonia under the temperature above 160 DEG C, and has the nitrogen selectivity of nearly 80 percent, good low-temperature oxidbillity, high catalytic efficiency and high stability. The oxidation catalyst contains porous inorganic oxide carrier, silver as an active component and cerium as an accessory ingredient, wherein the porous inorganic oxide carrier can be silicon dioxide, aluminium oxide, zirconia, lanthana, zinc oxide or titanium dioxide or the mixture of more than one of the oxide; the active component is formed by loading silver which a metal element with the weight conversion value of 1-15 percent on the inorganic oxide carrier; and the accessory ingredient is formed by simultaneously loading cerium and silver with the metal element weight conversion value of 5-50 percent on the inorganic oxide carrier.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
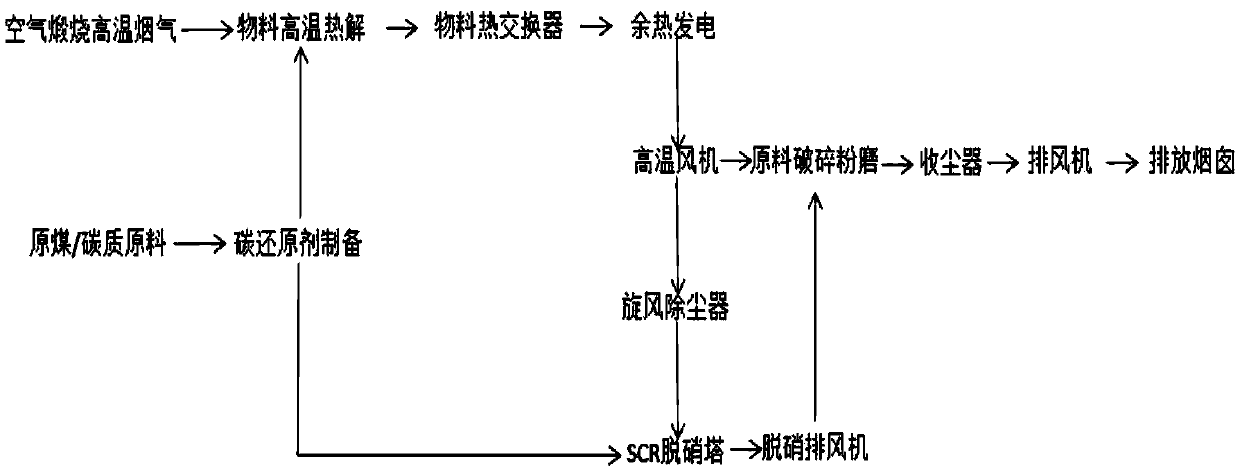
Method for reducing NOx in high-temperature calcination by SCR denitration technology adopting carbon-series reduction agent.
ActiveCN104174265AGuaranteed StrengthEasy to useNitrogen preparationDispersed particle separationReduction rateFlue gas
The invention provides a method for reducing NOx in high-temperature calcination by an SCR denitration technology adopting a carbon-series reduction agent. The method comprises the steps of preparing the carbon-series reduction agent, carrying out primary denitration burning, and then carrying out secondary denitration in an SCR catalyst tower. A gaseous carbon-series reduction agent mainly adopting CO and H2 is prepared, one part of the gaseous carbon-series reduction agent is used for carrying out primary high-temperature denitration at a high-temperature flue gas outlet in material calcination process to reduce the NOx in the high-temperature flue gas into inert N2NOx, and the reduction rate is about 30%; the other part of the gaseous carbon-series reduction agent is used for secondary catalytic denitration in the SCR denitration tower, and the reduction rate of the NOx is more than or equal to 50%. Compared with the prior art, the method provided by the invention has the advantages that the denitration reaction temperature is very low and is 220-410 DEG C, and excessive NO exists, so that the risk of escape of trace amount reduction gases such as CO and H2 together along with waste gas is greatly reduced; and the discharged waste gas subjected to denitration treatment passes through material crushing and grinding equipment, the residual heat of the waste gas is utilized for heating and drying materials, so that the energy-efficiency utilization rate of the whole system is increased.
Owner:凌庭生
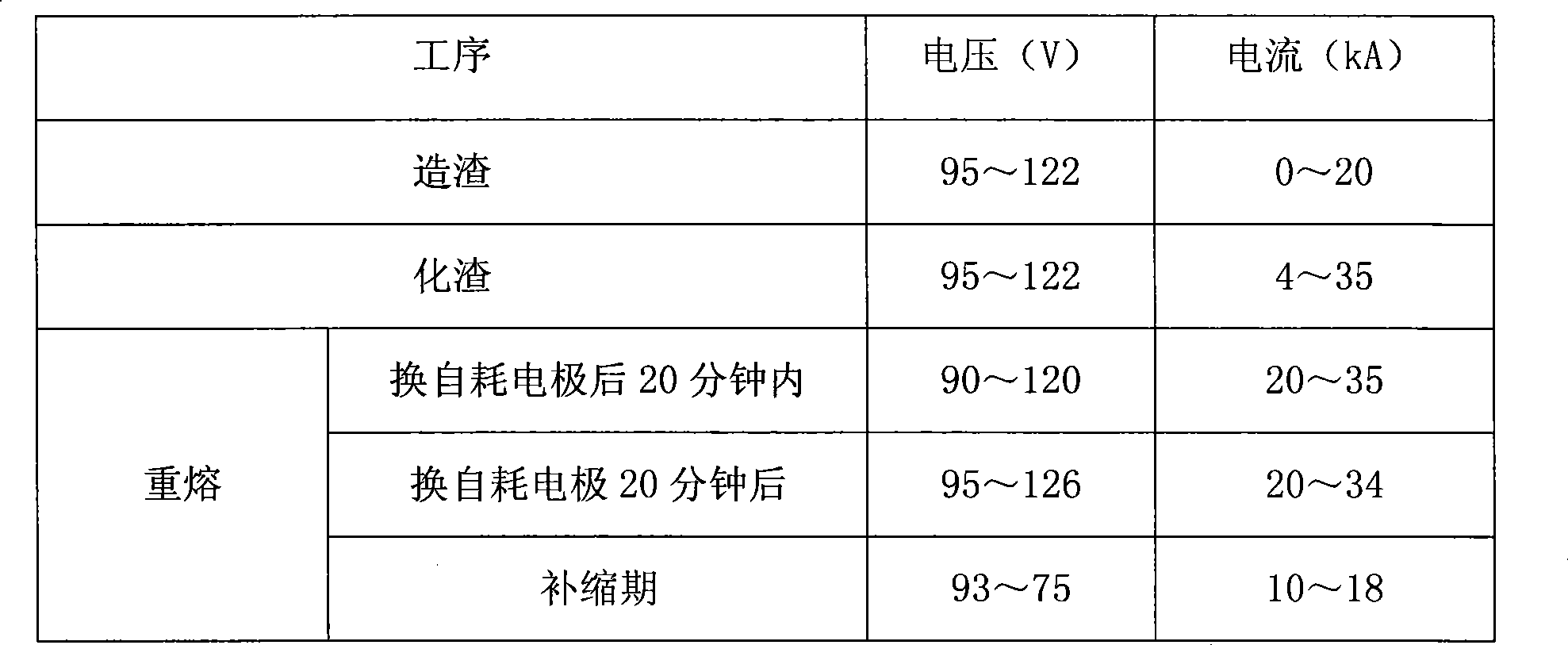
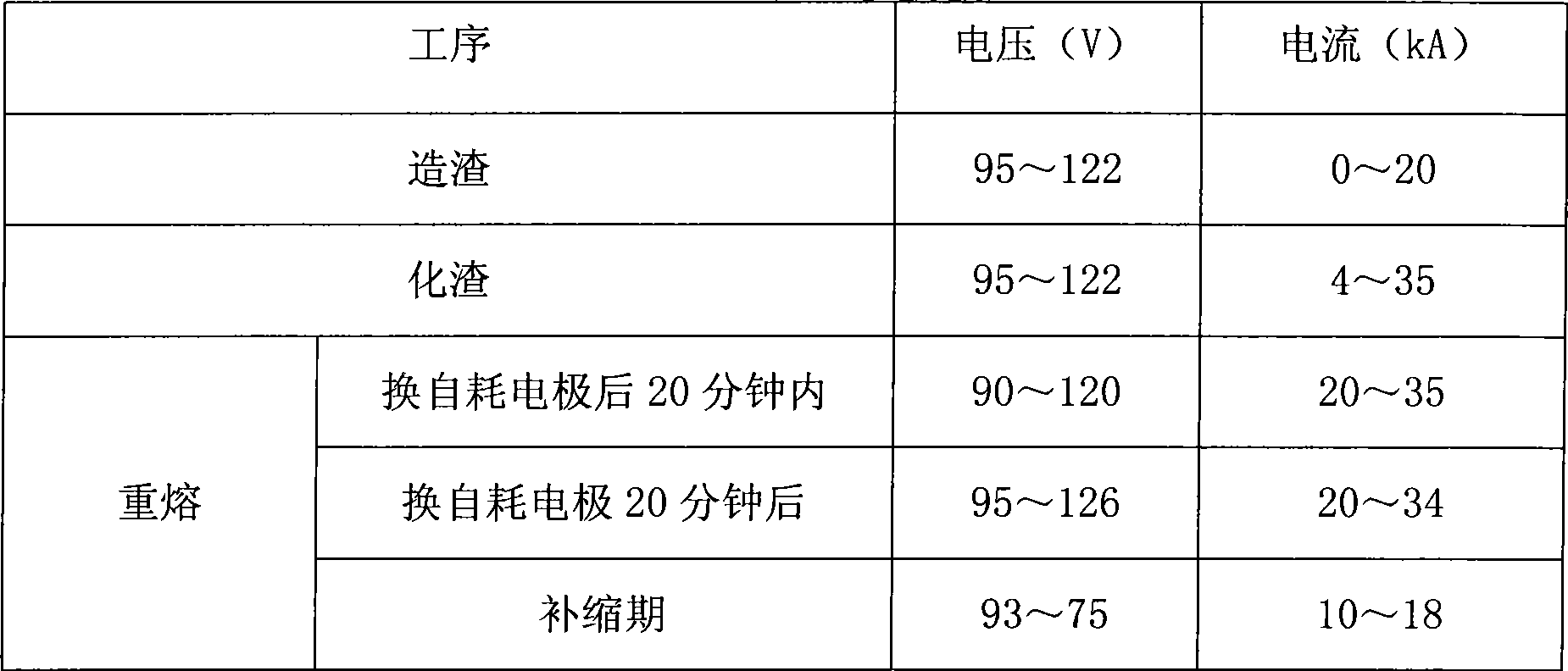
Method for manufacturing large-sized high purity 12Cr% low-ilicon low-aluminum electroslag remelting steel ingot
ActiveCN101469370AReduce or remove oxygen contentReduce use costNitrogen preparationSlagPositive pressure
The invention discloses a method for manufacturing a large-size high-purity 12Cr percent low-silicon low-aluminum electroslag remelting steel ingot, which comprises the concrete steps of: 1, preparation, in which nitrogen with the pressure of between 0.50 and 0.55MPa is aerated into a crystallizer; 2, refining melt-casting, in which the pressure of the nitrogen in the crystallizer is adjusted to ensure that the slag making is performed when the pressure of the nitrogen is between 0.45 and 0.5MPa, a graphite electrode is taken out after a slag material is entirely melted, the pressure of the nitrogen is adjusted to ensure that the pressure of the nitrogen is between 0.40 and 0.50MPa, and a consumable electrode is arranged for remelting; and 3, ingot stripping. The method utilizes the dry nitrogen which is aerated into the crystallizer in a large quantity during the electroslag remelting to replace air in the crystallizer to ensure that the pressure of the nitrogen in the crystallizer keeps positive pressure, thereby achieving the effect of reducing or removing the oxygen content in gas in a furnace during the electroslag remelting in similar manner. The method has the advantages of small investment, low application cost, and easy realization.
Owner:SHANGHAI ELECTRIC SHMP CASTING & FORGING CO LTD +1
Compositions of Matter Comprising Nanocatalyst Structures, Systems Comprising Nanocatalyst Structures, and Related Methods
InactiveUS20160030926A1Easy to set upLow costLiquid hydrocarbon mixture productionHydrogen/synthetic gas productionNano catalystNanofiber
Methods of forming and producing nanocatalysts mounted on or within nanofiber or nanotube structures are disclosed. The mounting structures prevent the nanocatalysts from agglomerating and retain the nanocatalysts in a reactor. The nanocatalysts may be grown over a bulk catalyst material without treating the nanotubes after forming the nanotubes. The resulting nanocatalysts remain catalytically active immediately after formation of the mounting supports and are effective in a wide variety of reactions. Systems are disclosed for reacting reaction gases to form mounting structures with at least one embedded nanocatalyst in the growth tips. The mounting structures may catalyze a different, subsequent reaction than the nanofiber formation reaction, which may take place in the same or a different reactor. Methods of forming a mass of nanocatalysts and catalyzing a reaction with the mass of nanocatalysts are disclosed. Systems are disclosed for forming a mass of nanocatalysts and catalyzing another reaction with the mass of nanocatalysts.
Owner:SEERSTONE
Copper-based supported ammoxidation catalyst and preparation method thereof
InactiveCN108043451AHigh selectivityReduce generationGas treatmentNitrogen preparationActive componentCopper
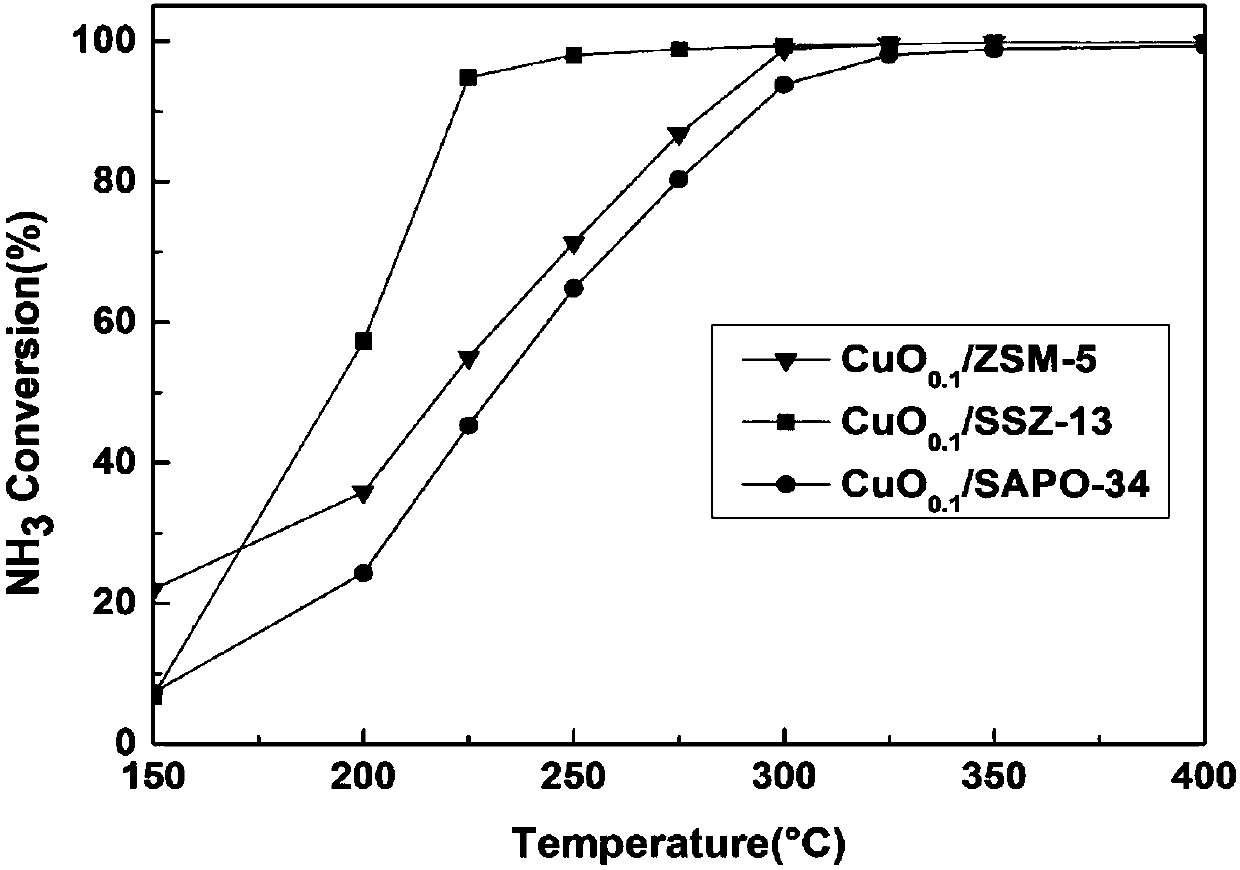
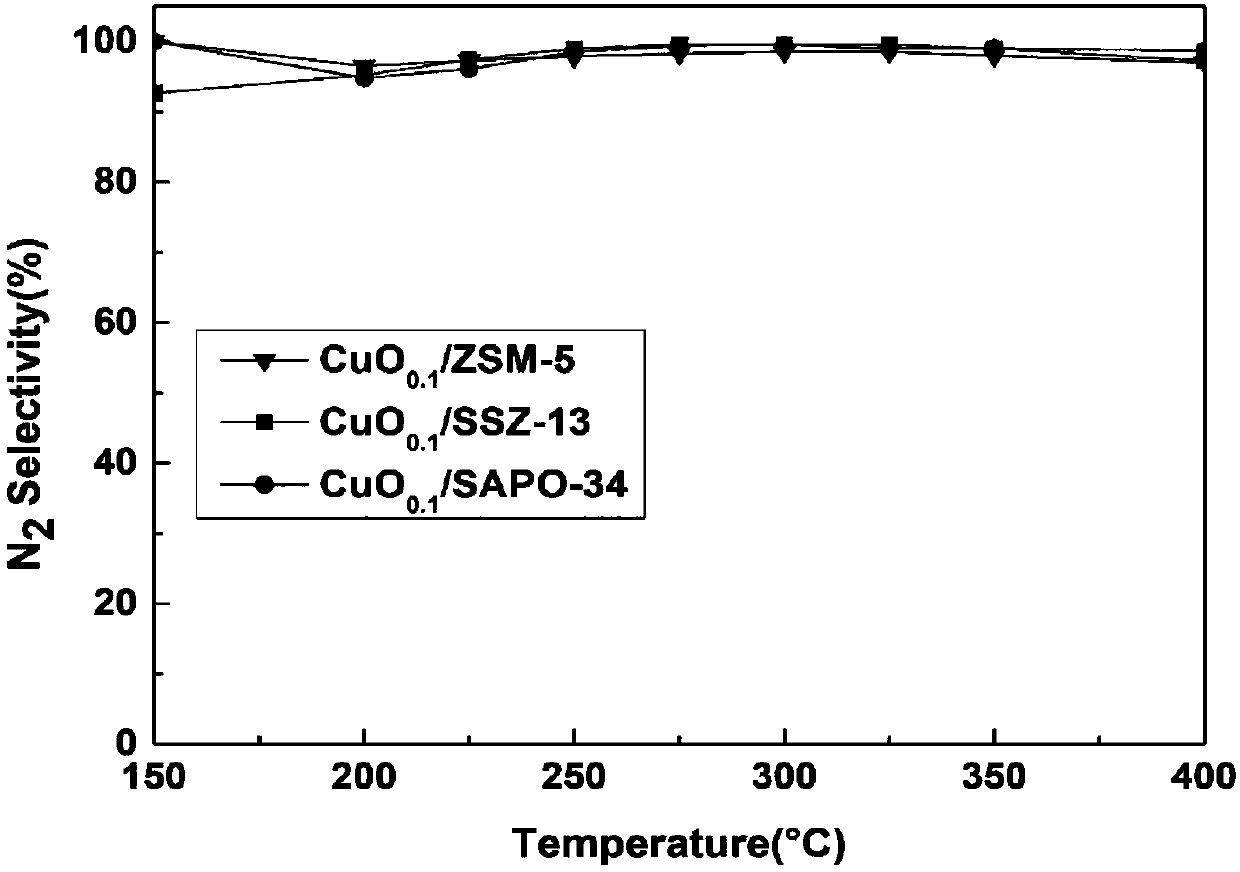
The invention discloses a copper-based supported ammoxidation catalyst and a preparation method thereof. The catalyst takes CuO as an active component and is expressed as CuO / M (M represents a supporter), wherein the supporting ratio is m(CuO) / m(M)=0.1. The invention also provides the preparation method of the copper-based supported ammoxidation catalyst. The prepared copper-based supported ammoxidation catalyst is applicable to ammonia gas purification for fixed sources and movable sources and can be applied in the downstream position of an SCR system to eliminate ammonia escape. Meanwhile, the catalyst is also applicable to treatment of ammonia gas produced and exhausted in breeding industry plants and agricultural sources. Compared with other ammoxidation catalysts, the catalyst copper-based supported ammoxidation catalyst taking SSZ-13 as a supporter has the characteristics of no toxicity, high activity, high selectivity and the like, good NH3 conversion rate is realized by the aidof the active component CuO, high Na selectivity is guaranteed by the aid of the supporter SSZ-13, and 90% or above of N2 selectivity can be kept in the whole temperature window. The copper-based supported ammoxidation catalyst has good catalytic activity and N2 selectivity in a wide temperature test interval at 200-400 DEG C.
Owner:TSINGHUA UNIV
Processes and uses of dissociating molecules
ActiveUS20110206593A1Toxic reductionMore degradableCyanogen compoundsElectrolysis componentsOxygenUltimate tensile strength
A process has been developed to selectively dissociate target molecules into component products compositionally distinct from the target molecule, wherein the bonds of the target molecule do not reform because the components are no longer reactive with each other. Dissociation is affected by treating the target molecule with light at a frequency and intensity, alone or in combination with a catalyst in an amount effective to selectively break bonds within the target molecule. Dissociation does not result in re-association into the target molecule by the reverse process, and does not produce component products which have a change in oxidation number or state incorporated oxygen or other additives because the process does not proceed via a typical reduction-oxidation mechanism. Target molecules include ammonia for waste reclamation and treatment, PCB remediation, and targeted drug delivery.
Owner:FAHS STAGEMYER
Microbial production of nitrous oxide coupled with chemical reaction of gaseous nitrous oxide
InactiveUS20100272626A1Minimize N2O productionProducer of clean energyWater treatment parameter controlOxygen/ozone/oxide/hydroxideChemical reactionAmmonia-oxidizing bacteria
A bioreactor designed to produce N2O from organic nitrogen and / or reactive nitrogen in waste is coupled to a hardware reactor device in which the N2O is consumed in a gas phase chemical reaction, e.g., catalytic decomposition to form oxygen and nitrogen gas. Heat from the exothermic reaction may be used to generate power. The bioreactor may use communities of autotrophic microorganisms such as those capable of nitrifier denitrification, ammonia oxidizing bacteria, and / or ammonia oxidizing archaea. A portion of the N2O dissolved in aqueous effluent from the bioreactor may be separated to increase the amount of gas phase N2O product. The amount of the gas phase N2O in a gas stream may also be concentrated prior to undergoing the chemical reaction. The N2O may alternatively be used as an oxidant or co-oxidant in a combustion reaction, e.g., in the combustion of methane.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Catalysts for NOX reduction employing h2 and a method of reducing NOX
InactiveUS20110150742A1Superior activity for production of ammoniaReduce storage performance requirementsNitrous oxide captureNitrogen preparationAmmoniaNOx
Owner:HEESUNG CATALYSTS CORP
Method of removing nitrogen oxides using an ilmenite material
InactiveUS6616904B1Easy to manageLess poisoningExhaust apparatusIsotope separationGas compositionInternal combustion engine
The invention relates to materials for removing the nitrogen oxides NO and NO2 present in exhaust gases, particularly from internal combustion engines of automotive vehicles running in a medium containing a superstoichiometric proportion of oxidizing agents, said materials being capable of absorbing the nitrogen oxides and of desorbing the nitrogen oxides when the temperature is raised, relative to the absorption temperature, or when the gas composition is changed to a rich mixture, said materials being mixed oxides in which the metals A and B are all octahedrally coordinated and are arranged to form the ilmenite structure ABO3. These materials absorb the nitrogen oxides and do not become poisoned in contact with the sulfur oxides and carbon oxides containing in the gases. In the presence of a group VIII metal, the material is capable of eliminating the adsorbed nitrogen oxides by reduction when the gas composition is changed to a rich mixture.
Owner:INST FR DU PETROLE
Carbon-supported transitional metal carbon nitride compound as well as preparation and application thereof
ActiveCN101411986AThe preparation method is simple and reliableGood dispersionPhysical/chemical process catalystsNitrogen preparationHydrogenCarbon nitride
The invention relates to a charcoal-supported transition metal carbon-nitrogen compound, a preparation method and application thereof. The transition metal compound is supported on a charcoal carrier by adopting a dipping method, and is dried and roasted to obtain a precursor. The prepared precursor is subjected to temperature programmed reaction in mixed gas of ammonia gas and hydrogen gas to obtain the transition metal carbon-nitrogen compound highly dispersed on the charcoal carrier. The charcoal-supported transition metal carbon-nitrogen compound prepared through the method shows good catalytic activity in various hydrogen-involved reactions.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalyst for ammonia decomposition, process for producing same, and method of treating ammonia
ActiveCN102159314ALow purityLow concentration to high purityGas treatmentNitrogen preparationDecompositionCobalt
The present invention relates to a catalyst for ammonia decomposition which is a catalyst for decomposing ammonia into nitrogen and hydrogen. The catalyst includes an active catalyst ingredient which comprises at least one transition metal selected from a group consisting of molybdenum, tungsten, vanadium, chromium, manganese, iron, cobalt, and nickel. It is preferred that (I) the active catalyst ingredient comprise at least one member selected from a group consisting of molybdenum, tungsten, and vanadium, or (II) the active catalyst ingredient comprise a nitride of at least one transition metal selected from a group consisting of molybdenum, tungsten, vanadium, chromium, manganese, iron, cobalt, and nickel, or (III) the active catalyst ingredient contain at least one iron-family metal selected from a group consisting of iron, cobalt, and nickel and further contain a metal oxide. This catalyst enables ammonia to be efficiently decomposed into nitrogen and hydrogen at a relatively low temperature and a high space velocity, whereby high-purity hydrogen is acquired.
Owner:NIPPON SHOKUBAI CO LTD
Preparation method of low-temperature catalyst for selective catalytic reduction of nitrogen oxide
ActiveCN101507920AHigh particle strengthTolerance to interferenceNitrogen preparationDispersed particle separationMaterials preparationFlue gas
The invention provides a method for preparing a catalyst which can selectively catalyze and reduce nitrogen oxides at a low temperature, and belongs to the technical field of material preparation and waste gas treatment. In the method, chromium and cerium are taken as main components, and the catalyst which can effectively catalyze NH3 and reduce NOx into N2 at a low temperature is obtained by the coprecipitation method or dipping method and burning the main components; the catalyst has high low-temperature activity, and can be used at a temperature of between 80 and 150 DEG C, and can also tolerate the interference of as high as 6 percent of CO in the system, so that the catalyst has better application prospect in the denitration operation of sintered flue gas.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Urea based composition and system for same
InactiveUS7140187B2Low toxicitySafe handlingGas turbine plantsFused electrolyte fuel cellsNitrogenNitrogen gas
Owner:AMENDOLA STEVEN C
Pyrite cinder separation, recovery and recycling method
InactiveCN105984893ASave landSaving and efficient useCalcium/strontium/barium carbonatesSolid waste disposalIron(II) oxideAluminium hydroxide
The invention discloses a pyrite cinder separation, recovery and recycling method and belongs to the technical field of an inorganic chemistry industry. The method comprises carrying out suspended separation on calcium hydroxide and alumina through water, dissolving and separating alumina through an ammonium salt, dissolving iron sesquioxide / ferrous oxide in the pyrite cinder through strong phosphoric acid, filtering to separate the mixture of sulfur, phosphorus and silica insoluble with strong phosphoric acid from iron sesquioxide / ferrous oxide, and separating and recovering resource materials such as iron sesquioxide / ferrous oxide and alumina or aluminum hydroxide. The separated product can be used for an industrial raw material and agricultural fertilizer.
Owner:耿兆翔
Method and device for synchronously removing sulfur dioxide and nitric oxide by flue gas biological method
InactiveCN102989272AEasy to handleLow costProductsNitrogen preparationVulcanizationChemical oxygen demand
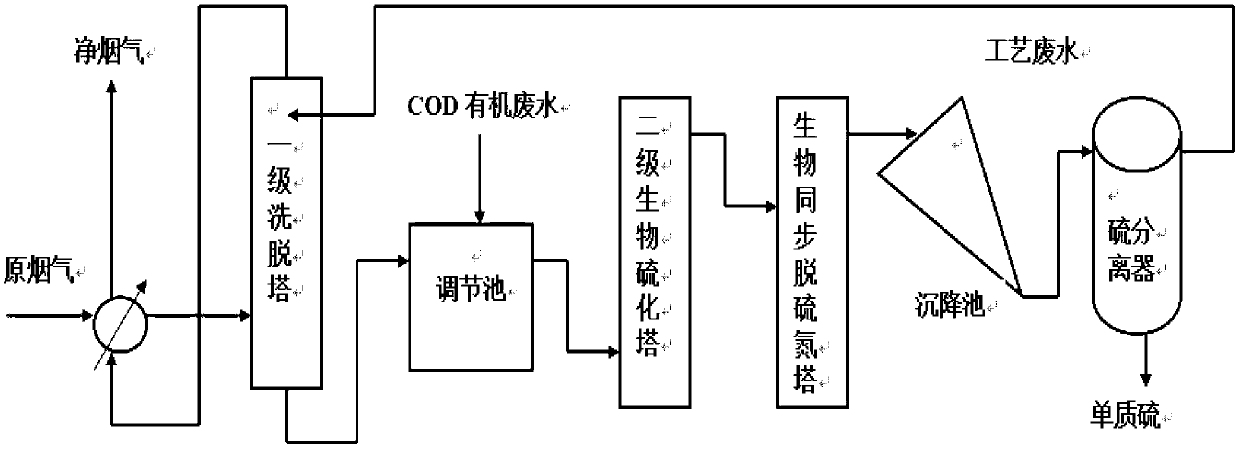
The invention relates to a method and a device for synchronously removing sulfur dioxide and nitric oxide by a flue gas biological method. The device is formed by connecting a primary eluting tower, an adjusting tank, a secondary biological vulcanization tower, a biological synchronous desulfurization and denitrification tower, a sedimentation tank and a sulfur separator in series. A flue gas purification treatment technology has low inflowing air requirements, is not afraid of dust and heavy metal and does not need strict pre-treatment. Corresponding microorganisms are added into a biological flora to effectively remove heavy metal. Meanwhile, a synchronous desulfurization and denitrification flora is autotrophic bacteria and has certain removing effect on CO2. An operation condition is moderate and the equipment does not need to be antiseptic. Besides, an external substance such as COD (Chemical Oxygen Demand) organic wastewater, only few microorganism nutrient substances and supplementing alkali for adjusting and controlling a pH (Potential of Hydrogen) value in the eluting tower need to be added; and a temperature needed by a rear-end biological reaction is maintained through heat obtained by front-end heat exchange. In a process, large-sized power consumption equipment is not needed; and the device and the method have the advantages that electric power consumption and water consumption are low compared with a conventional treatment process. Compared with the conventional cost, the cost of the whole process is 1 / 3 that of the conventional process, and the industrial production can be realized.
Owner:TIANJIN UNIV
Low energy consumption water-air separation device and method
ActiveCN104841237AHas a placeholder effectImprove separation efficiencyNitrogen preparationDispersed particle separationDecompositionEnergy expenditure
The invention discloses a low energy consumption water-air separation device and method. The device comprises a compressor, a pre-cooling system and at least one hydration tower, one decomposition tower, one vacuum pump and one heating pump; the compressor and the pre-cooling system are connected through a pipeline, the pre-cooling system is communicated with the air inlet of a first hydration tower, the liquid inlet of the first hydration tower is connected to a first vacuum pump, the hydrate liquid outlet of the first hydration tower is connected to the feed port of the first decomposition tower, the residual liquid outlet of the first decomposition tower is connected to the liquid inlet of the first hydration tower, the first hydration tower and the first decomposition tower are connected through a first heating pump, the upper portion of the first hydration tower is provided with a gas release recovery port, and the upper portion of the first decomposition tower is provided with an oxygen-enriched air release and recovery port. The air is separated according to the principle that hydration of oxygen and water is performed more easily as compared with that of nitrogen and water, the hydration pressure is lowered by adding hydration accelerator, the pressure difference for oxygen generating hydrate is larger than that for nitrogen, and the energy consumption can be reduced by more than 30%.
Owner:SOUTH CHINA UNIV OF TECH
Extinguishment method of fire-fighting truck with movable nitrogen making machine group and equipment thereof
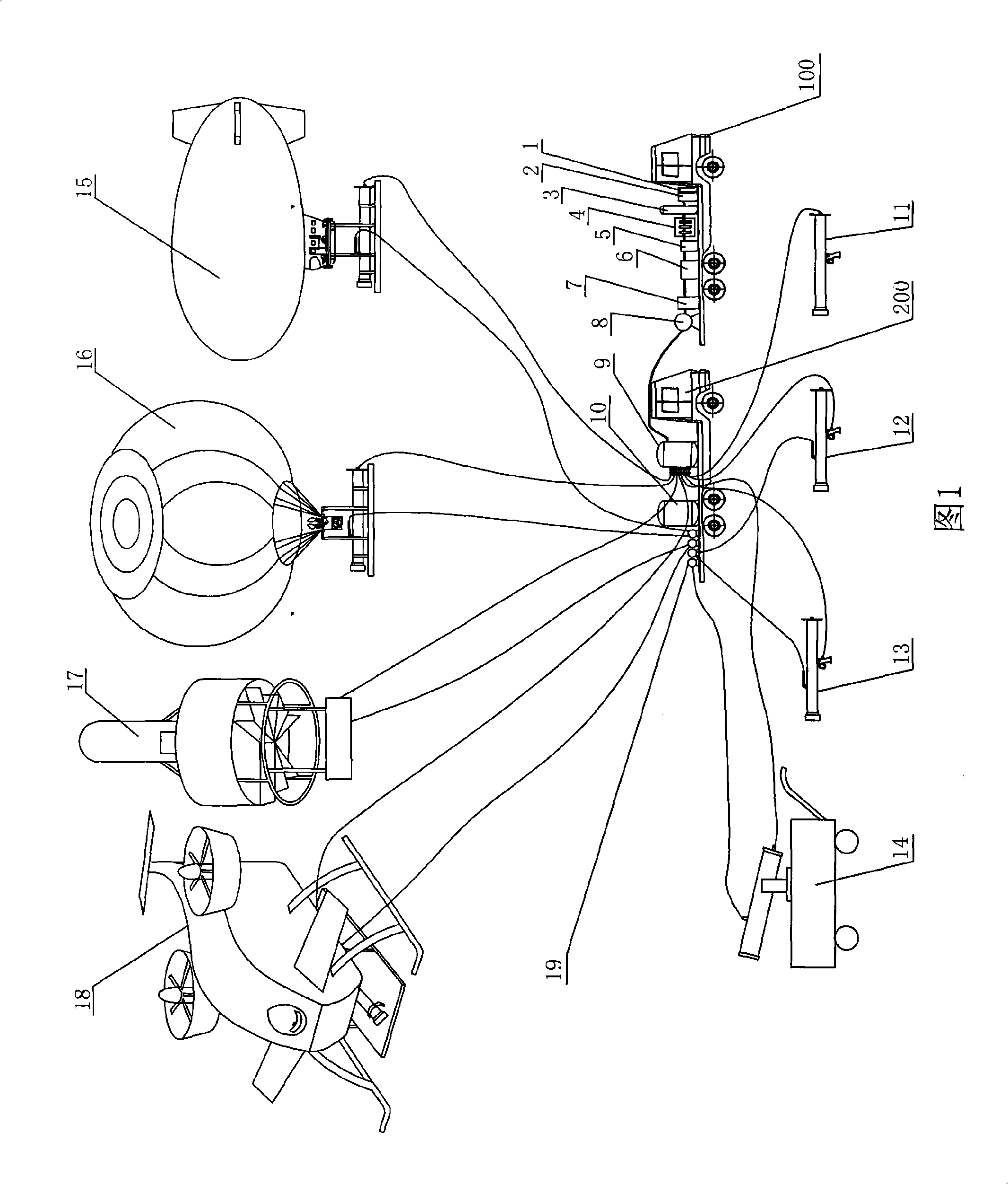
InactiveCN101301515AStable pressureEasy to useNitrogen preparationFire rescueNitrogen generatorExtinction
The invention relates to a fire-extinguishing method and a fire-extinguishing device for a mobile nitrogen generator fire engine, wherein, nitrogen is prepared by a fixedly arranged or connected device on a mobile nitrogen generator nitrogen preparation vehicle and then inputted to a nitrogen storage tank of a multifunctional fire-fighting vehicle; the nitrogen storage tank and a water storage tank are arranged on multifunctional fire-fighting vehicle; the nitrogen storage tank is connected with various branched air pipes; the water storage tank is connected with a pump unit which is connected with the various branched water pipes; and the water pipes and the air pipes are respectively connected to a pulse air-blast spray nozzle, a two-phase flow water fog fire-fighting gun, a pulse water cannon, a fire-fighting airship, a fire-fighting fire balloon, an unmanned fire-fighting rotary wing aircraft and a fire helicopter. The fire-extinguishing method and the fire-extinguishing device take nitrogen as extinguishing agent and the nitrogen preparation device fixed on the fire engine as nitrogen source to perform nitrogen fire extinction on the fire scene, which can not pollute the surrounding environment and can not generate secondary disasters. The fire-extinguishing method and the fire-extinguishing device are capable of realizing multifunctional large-range solid fire-fighting and are suitable for large-range fire scenes.
Owner:BEIJING SMHW MAGNETIZATION TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com