Copper-based supported ammoxidation catalyst and preparation method thereof
A supported, ammonia oxidation technology, applied in the field of ammonia escape control and environmental protection, to achieve the effects of high selectivity, improved N2 selectivity, and high activity selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
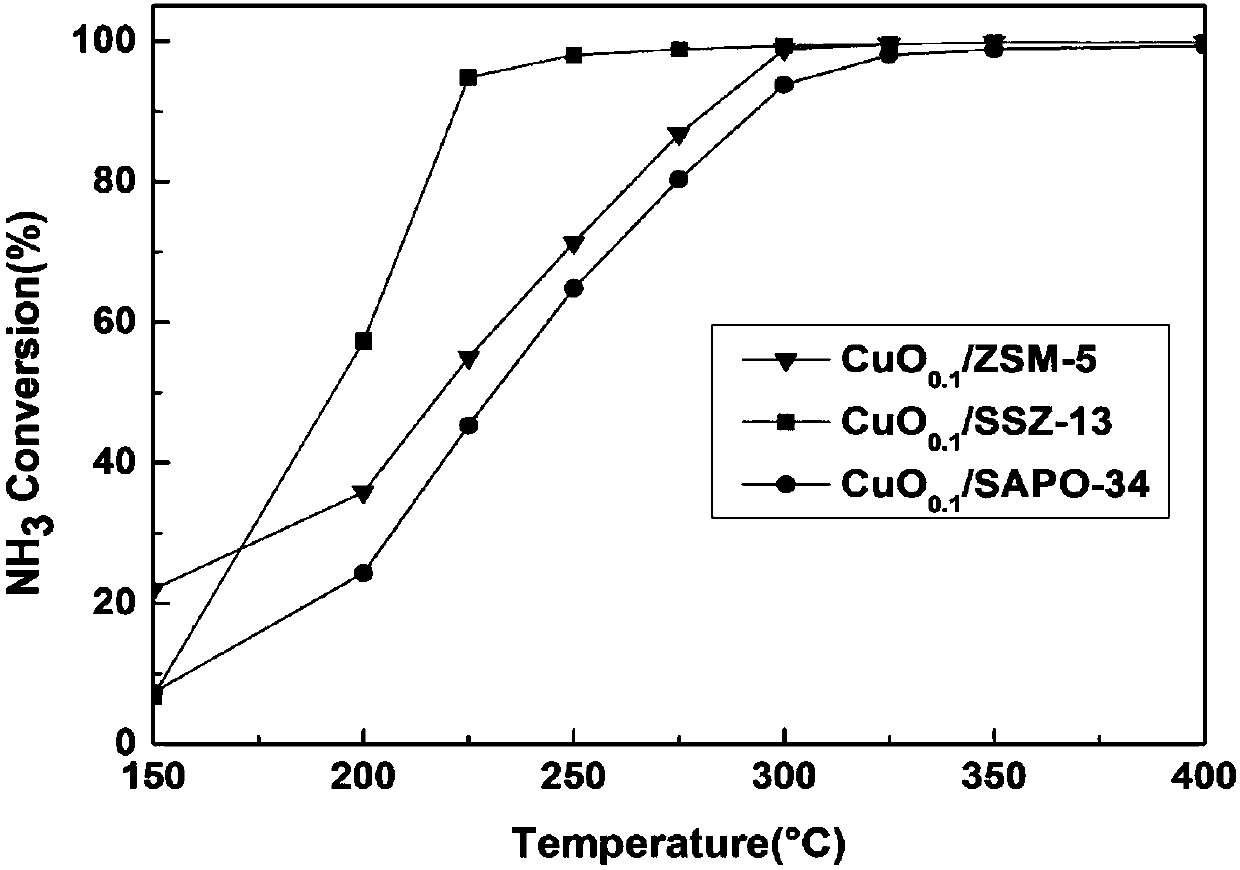
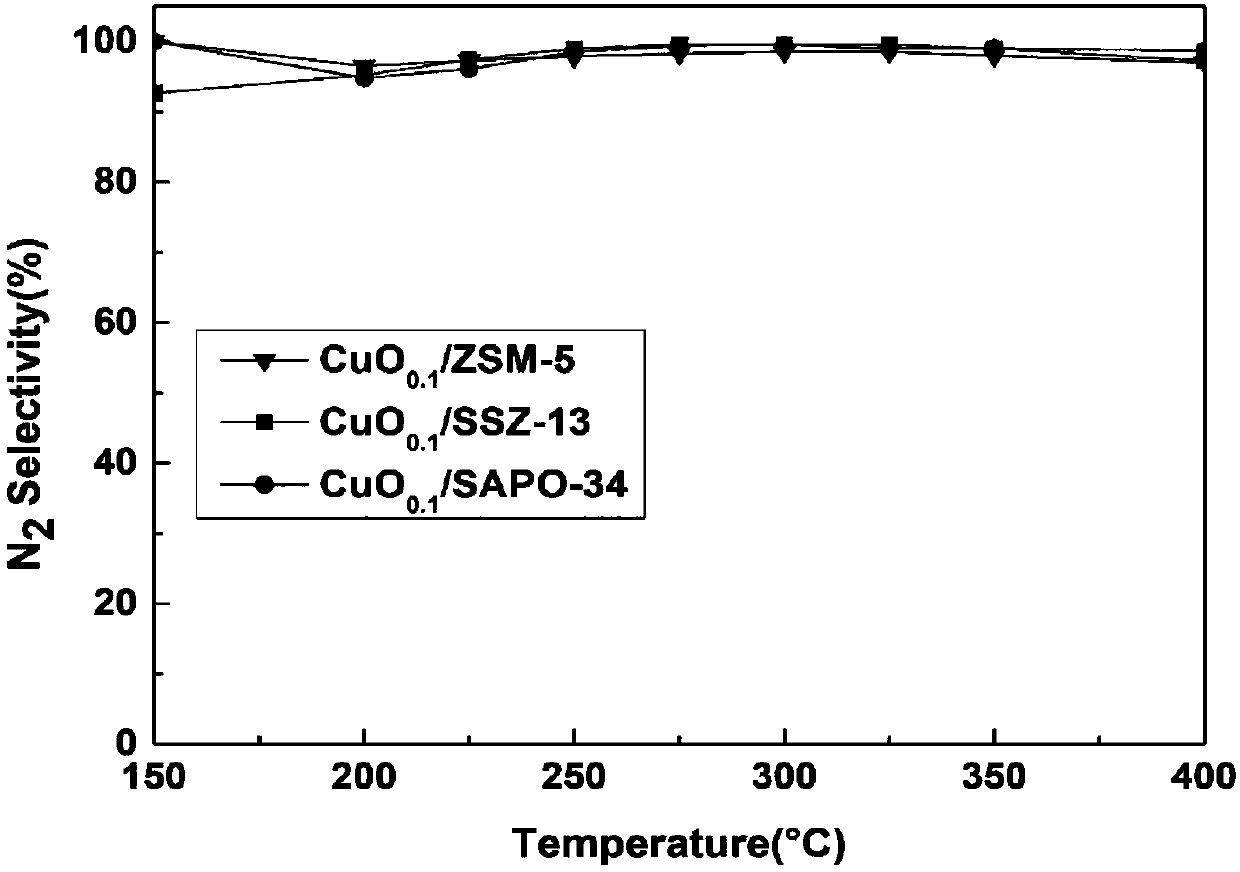
Image
Examples
Embodiment 1
[0027] CuO 0.1 / Preparation of ZSM-5 samples
[0028] (1) take by weighing 0.9075g copper nitrate trihydrate and 3g ZSM-5 powder for subsequent use;
[0029] (2) Dissolve the weighed copper nitrate trihydrate in a small amount of deionized water;
[0030] (3) After the copper nitrate trihydrate is completely dissolved, add the weighed ZSM-5 powder therein, and stir at room temperature for 5 minutes;
[0031] (4) After stopping stirring, transfer the obtained solution to a rotary evaporator, and use a rotary evaporator to evaporate at a speed of 500r / min and a water bath temperature of 70°C for 2h to evaporate the solvent;
[0032] (5) Put the evaporated material into an oven at 100°C for 12 hours to obtain a semi-finished product;
[0033] (6) Put the obtained semi-finished product into the muffle furnace, and heat it at 10°C min -1 The temperature was raised to 400°C for 6 hours, and then cooled naturally in the furnace to prepare a copper-based supported ammoxidation ca...
Embodiment 2
[0036] CuO 0.1 / SAPO-34 sample preparation
[0037] (1) Take by weighing 0.9075g copper nitrate trihydrate and 3g H-SAPO-34 powder for subsequent use;
[0038] (2) Dissolve the weighed copper nitrate trihydrate in a small amount of deionized water;
[0039] (3) After the copper nitrate trihydrate is completely dissolved, add the weighed H-SAPO-34 powder therein, and stir at room temperature for 5 minutes;
[0040] (4) After stopping stirring, transfer the obtained solution to a rotary evaporator, and use a rotary evaporator to evaporate at a speed of 400r / min and a water bath temperature of 60°C for 2h to evaporate the solvent;
[0041] (5) Put the evaporated material into an oven at 100°C and dry for 14 hours to obtain a semi-finished product;
[0042] (6) Put the obtained semi-finished product into the muffle furnace, and heat it at 10°C min -1 The temperature was raised to 500°C for 6 hours, and then cooled naturally in the furnace to prepare a copper-based supported am...
Embodiment 3
[0045] CuO 0.1 / SSZ-13 sample preparation
[0046] (1) Take by weighing 0.7500g copper acetate trihydrate and 3g H-SSZ-13 powder for subsequent use;
[0047] (2) Dissolve the weighed copper acetate trihydrate in a small amount of deionized water;
[0048] (3) After the copper acetate trihydrate is completely dissolved, add the weighed H-SSZ-13 powder, and stir at room temperature for 5 minutes;
[0049] (4) After stopping stirring, transfer the obtained solution to a rotary evaporator, and use a rotary evaporator to evaporate at a speed of 500r / min and a water bath temperature of 60°C for 4 hours to evaporate the solvent;
[0050] (5) Put the evaporated material into an oven at 100°C for 12 hours to obtain a semi-finished product;
[0051] (6) Put the obtained semi-finished product into the muffle furnace, and heat it at 10°C min -1 The temperature was raised to 400°C for 8 hours, and then cooled naturally in the furnace to prepare a copper-based supported ammoxidation cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com