Catalyst for ammonia decomposition, process for producing same, and method of treating ammonia
A catalyst, ammonia decomposition technology, applied in catalyst activation/preparation, separation methods, chemical instruments and methods, etc., can solve the problems of high catalyst performance, low space velocity, low ammonia concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] "The preparation method of ammonia decomposition catalyst (I)"
[0063] Hereinafter, preferred specific examples of the method for producing the ammonia decomposition catalyst (I) of the present invention will be given, but the method is not limited to the following production method as long as the subject of the present invention can be achieved.
[0064] (1) An oxide of component A, a mixture of an oxide of component A and an oxide of component B, a composite oxide of component A and component B, a mixture of oxides of component C added to these, or a mixture of these A method in which a calcined product obtained by calcining the aqueous solution of the C component and drying the mixture is used as a catalyst;
[0065] (2) A method of further treating the calcined product of (1) at a temperature of 300-800° C. with ammonia gas or nitrogen-hydrogen mixed gas (nitridation treatment);
[0066] (3) A method of roasting an aqueous solution of a salt containing component A...
Embodiment
[0146] Hereinafter, the present invention will be described in more detail by citing experimental examples, but the present invention is not limited to the following experimental examples, and appropriate changes can be added to the range appropriately obtained from the gist described before and after. Implementation, these are included in the technical scope of the present invention.
[0147] Ammonia decomposition catalyst (I)
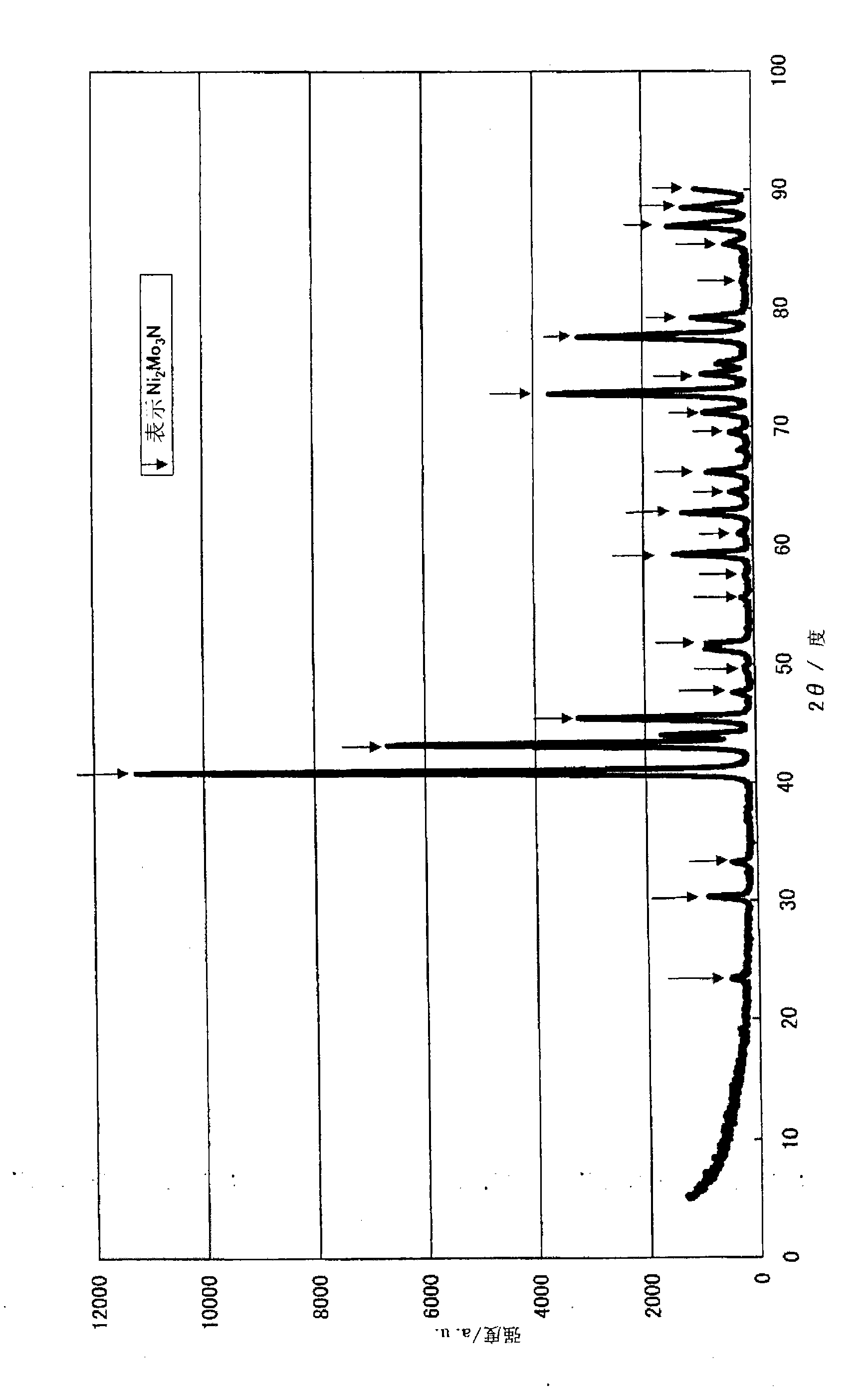
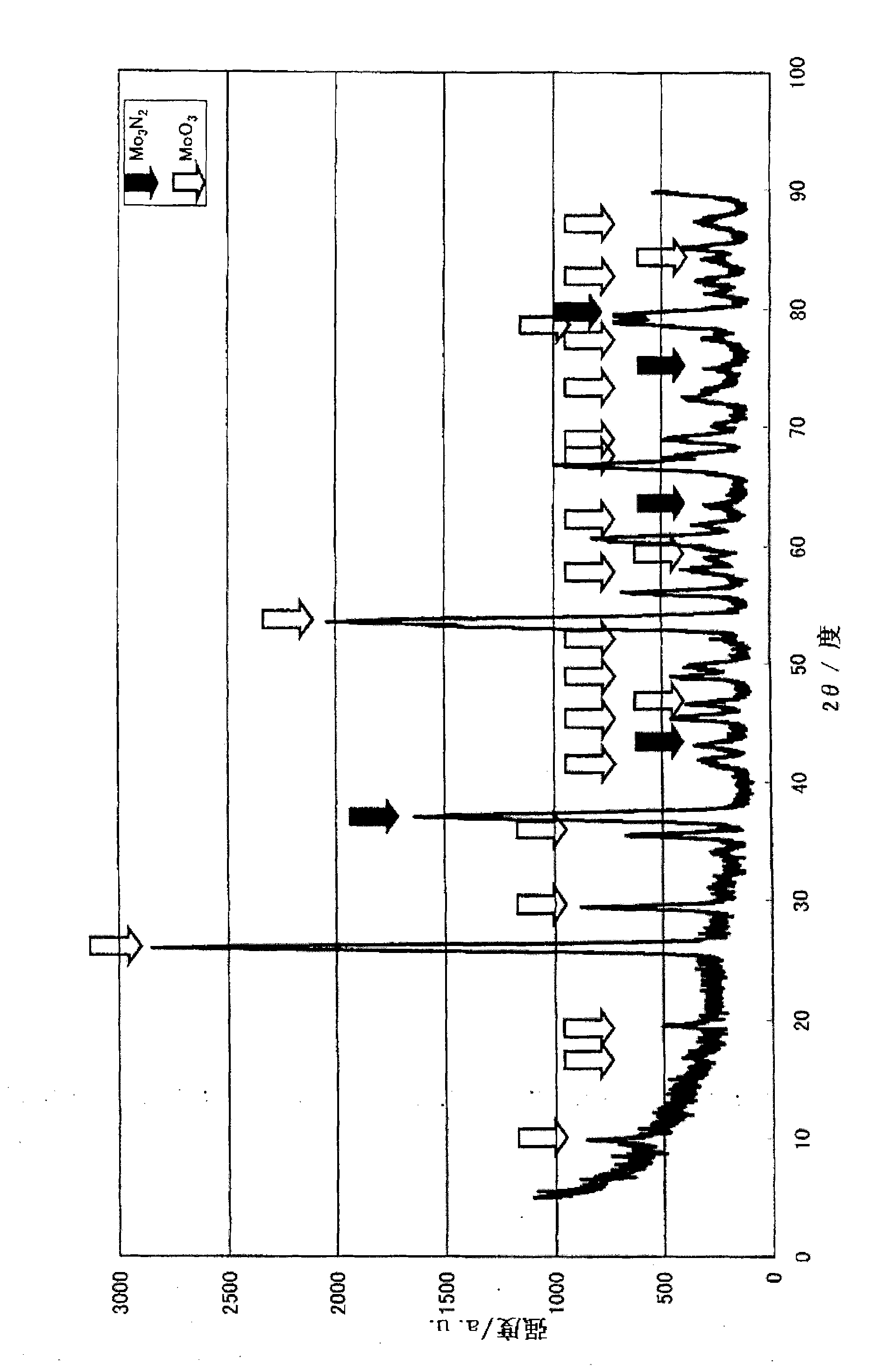
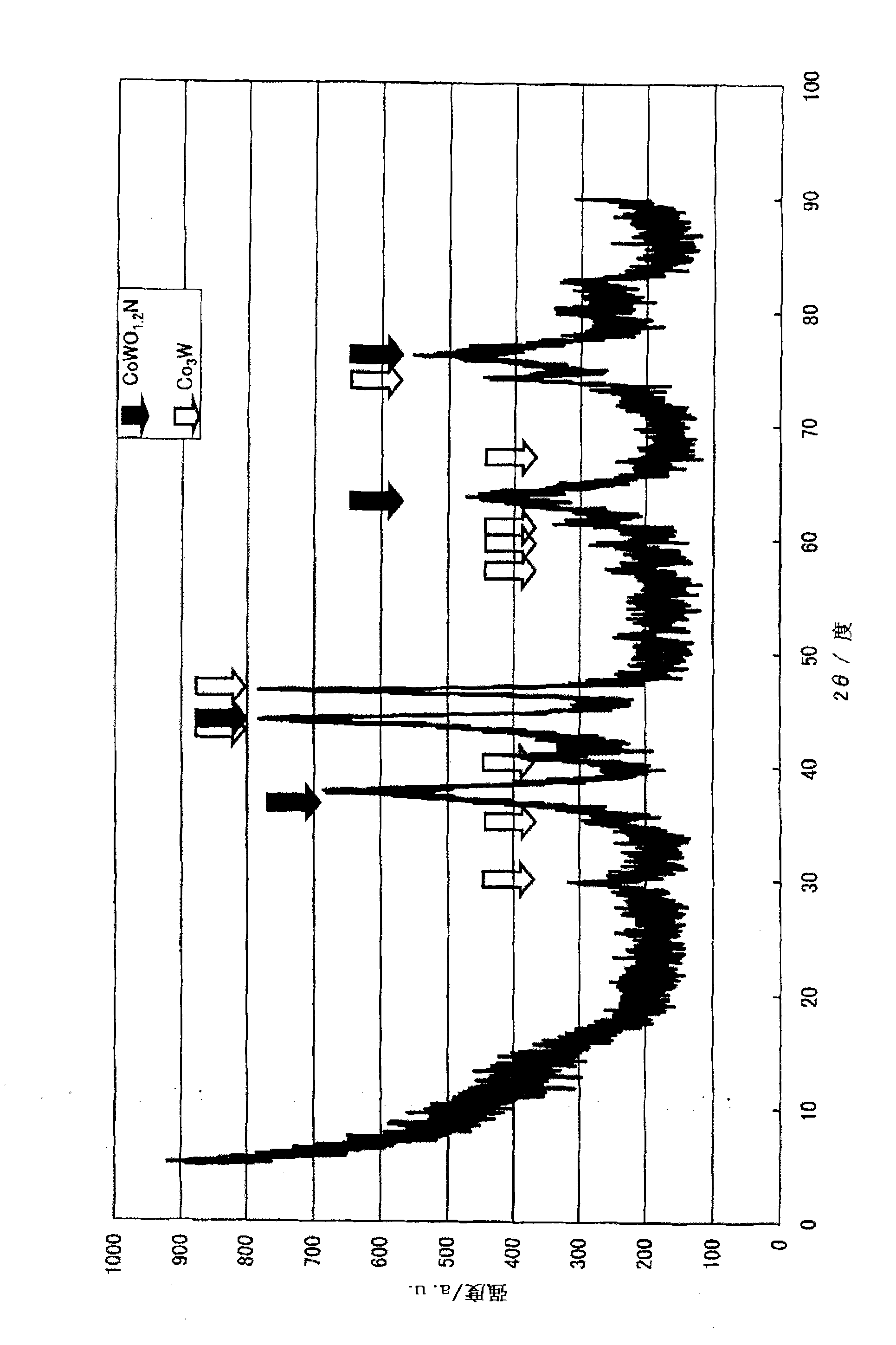
[0148] First, a production example and performance evaluation of the ammonia decomposition catalyst (I) will be described. In addition, an X-ray diffractometer (product name "RINT-2400", manufactured by Rigaku Corporation) was used for the X-ray diffraction measurement. The X-ray source uses CuKα (0.154nm), and it is carried out under the following measurement conditions, that is, the X-ray output power is 50kV, 300mA, the divergence slit is 1.0mm, and the divergence slit length (dissipation limit slit) is 10mm, The scanning speed is 5 degrees per m...
experiment example I-1
[0150] Dissolve 80.00 g of cobalt nitrate hexahydrate in 400.00 g of distilled water. Separately, 48.53 g of ammonium molybdate was slowly added and dissolved in 250 g of boiling distilled water. After mixing the two aqueous solutions, heat and stir, and evaporate to dryness. The obtained solid was dried at 120° C. for 10 hours, then baked at 350° C. for 5 hours under a nitrogen stream, and 3 hours at 500° C. under an air stream. Confirmed as α-CoMoO by X-ray diffraction measurement 4 .
[0151] Furthermore, 0.5-1.0 mL of α-CoMoO was filled in a reaction tube made of SUS316 4 , the temperature was raised to 400° C. under nitrogen gas (hereinafter referred to as “nitrogen”) at a rate of 30-50 mL / min. Then, the temperature was raised to 700° C. under feeding ammonia gas (hereinafter referred to as “ammonia”) at 50-100 mL / min, and the treatment (nitridation treatment) was carried out at 700° C. for 5 hours to obtain an ammonia decomposition catalyst (hereinafter referred to a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com