Carbon-supported transitional metal carbon nitride compound as well as preparation and application thereof
A carbonitride and transition metal technology, applied in the field of supported transition metal carbonitride and its preparation, can solve problems such as single nitride or carbide, and achieve the effects of low preparation cost, simple preparation process and easy molding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] WN x C y Preparation of / AC
[0028] Weigh 0.62g, 1.10g, 2.75g, 4.88g of ammonium metatungstate and dissolve them in 6.0ml of deionized water respectively, then impregnate equal volumes of the obtained clear solutions onto 4.0g of activated carbon (AC) carriers, and dry at room temperature for 12 hours, then dried in an oven at 120°C for 12 hours, and fired at 500°C for 4 hours under a nitrogen atmosphere to obtain WO 3 / AC precursor. WO 3 / AC precursor, temperature programmed reaction in ammonia gas, the space velocity is 5000 / hr, from room temperature to 450 °C at a rate of 10 °C / min, and then raised to 800 °C at a rate of 1 °C / min. After keeping at the temperature for 2 hours, cool to room temperature to prepare WN with active component mass content of 10, 16.7, 33.3, 55 wt%. x C y / AC Catalyst.
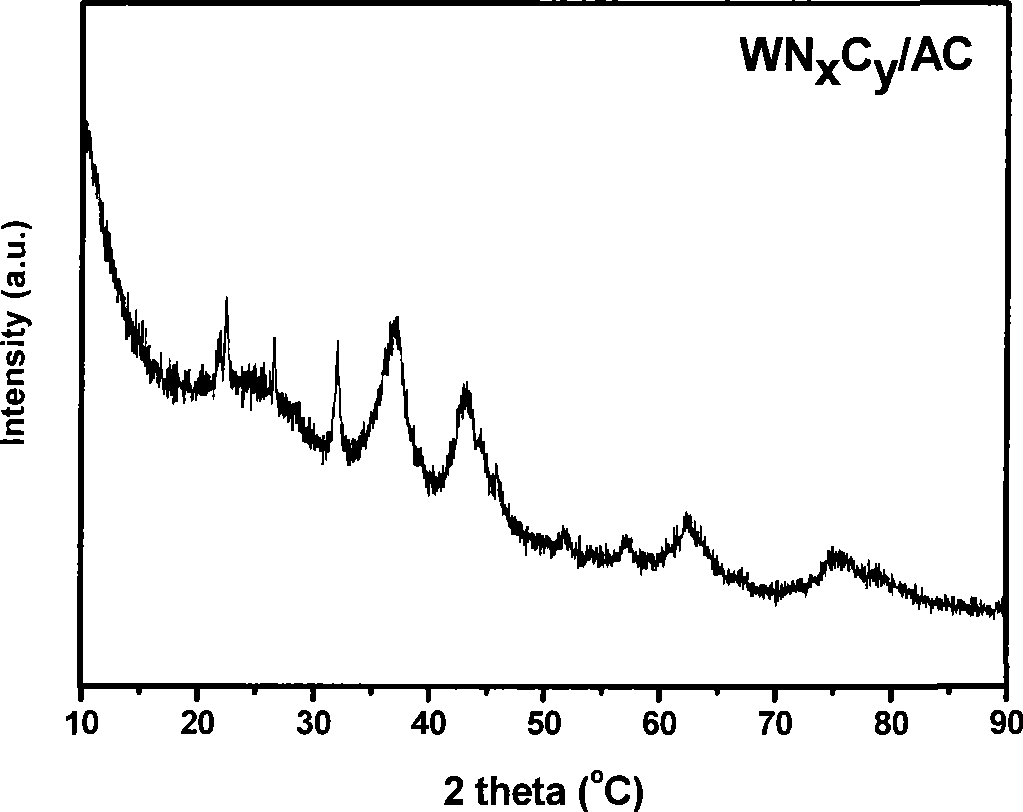
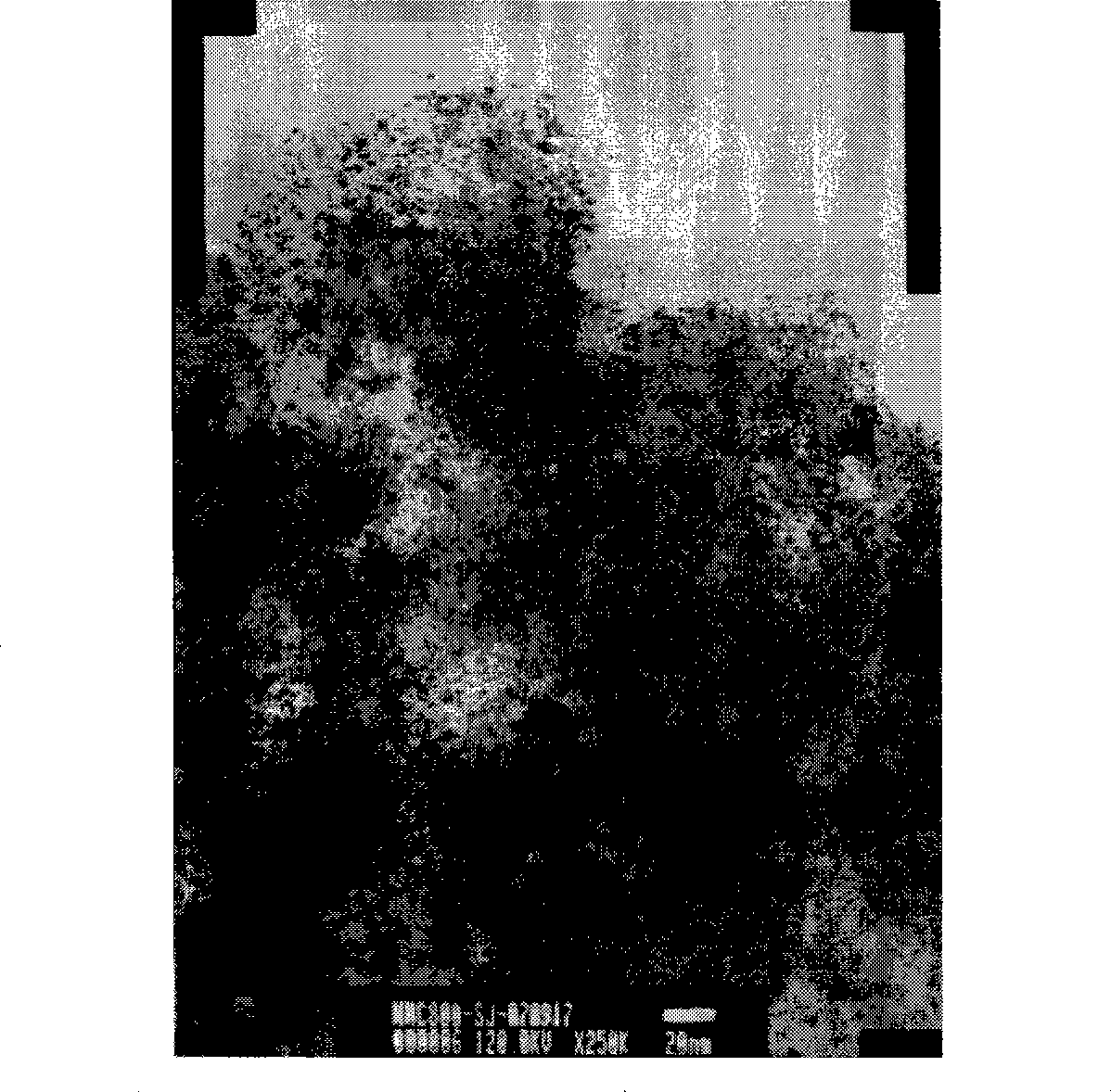
[0029] Active ingredient 16.7wt% WN prepared as above x C y The results of X-ray diffraction and transmission electron microscopy of the / AC catalyst are as follow...
Embodiment 2
[0031] WN at different temperatures x C y Preparation of / AC (active component 16.7wt%)
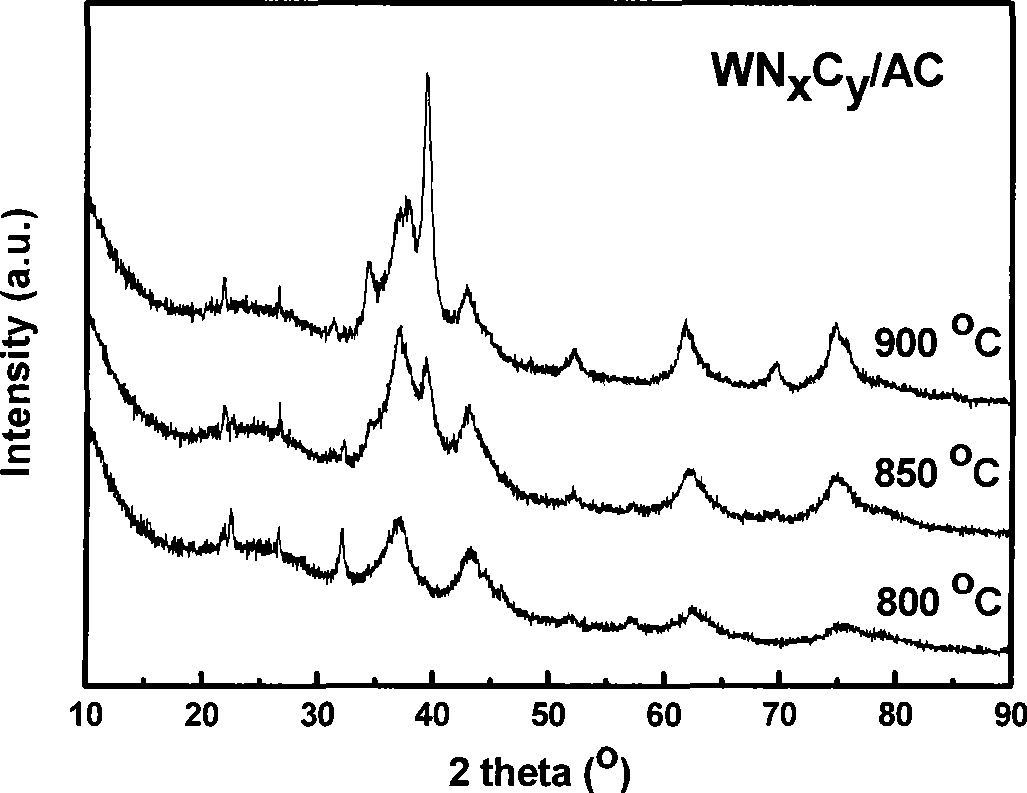
[0032] The difference from Example 1 is that WN was prepared at final preparation temperatures of 800°C, 850°C and 900°C, respectively x C y / AC (16.7 wt%) catalyst. The X-ray diffraction results of the obtained sample are as follows image 3 shown. It can be seen from the figure that WN is produced at 800°C x C y Catalyst, WN can be produced when the temperature rises above 850°C x C y and W 2 C mixed-phase catalyst.
Embodiment 3
[0034] WN at different airspeeds x C y Preparation of / AC (active component 16.7wt%)
[0035] The difference from Example 1 is that the space velocity of ammonia is 2500hr respectively -1 , 5000hr -1 , 10000hr -1 WN was produced under the condition x C y / AC (16.7 wt%) catalyst. The X-ray diffraction results of the obtained sample are as follows Figure 4 shown. From Figure 4 It can be seen that WN can be obtained at different airspeeds x C y / AC, increase the preparation space velocity, which is beneficial to WN x C y Dispersion on the surface of activated carbon.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com