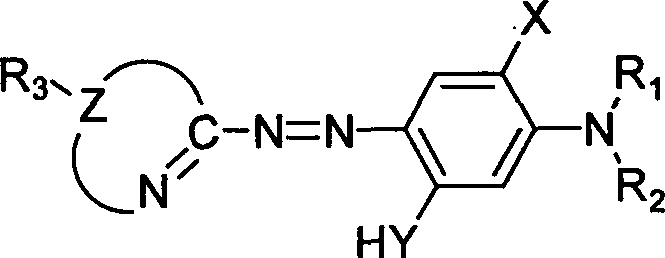
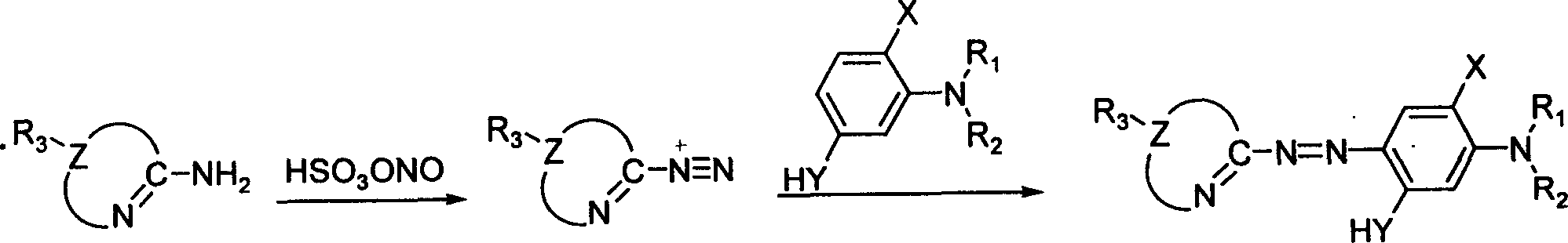
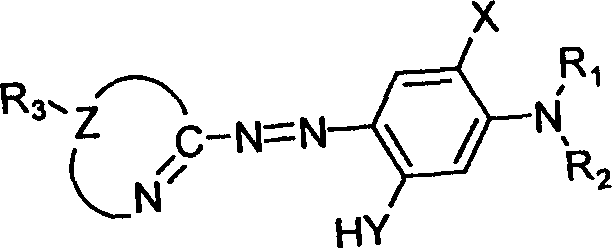
Heterocyclyl-azo-dialkyl aminophenol pigments and process for synthesizing the same
A technology of an alkylaminophenol and a synthesis method, applied in the directions of azo dyes, monoazo dyes, organic dyes, etc., can solve the problems of inconvenient laboratory operation, difficult to fully contact, increased product separation and purification, and easy to achieve reaction conditions. Control, excellent optical properties, simple instrument effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Dissolve 0.4045 g (4.0 mmol) of the diazo component 2-amino-1,3,4-thiadiazole in 4 ml of acetic acid and 2 ml of propionic acid, then stir, at -10-5°C, Add 0.4 ml of sulfuric acid, slowly add 1.22 g (4.8 mmol) of 50% nitrosyl sulfuric acid, and carry out a diazotization reaction for 2 hours to prepare a diazonium salt solution.
[0017] (2) Dissolve 0.6035 g (4.4 mmol) of the coupling component N, N-dimethyl-3-aminophenol, 0.16 g of urea and 1.6 g of sodium acetate in 20 ml of methanol, cool to -10-5 ° C, and stir , slowly drop the above diazonium salt solution into the reaction system, continue to stir for 4 hours, let stand overnight, filter, wash with water, and dry to obtain the azo dye 2-[2-(1,3,4-thiadiazolyl) Azo]-5-(N,N-dimethylamino)phenol 0.788 grams.
[0018] Yield: 79%, melting point: 241-242°C.
[0019] The characteristic absorption peaks of infrared spectrum (FT-IR) are: 3075, 2955, 2928, 2872, 1637, 1534, 1471, 1318, 1289, 1195, 1143, 1072, 895, 808...
Embodiment 2
[0029] (1) Dissolve 0.4606 g (4.0 mmol) of the diazo component 2-amino-5-methyl-1,3,4-thiadiazole in 4 ml of acetic acid and 2 ml of propionic acid, then stir, at -10-5 Under the condition of ℃, add 0.4ml sulfuric acid, slowly add 1.22 grams (4.8mmol) of 50% nitrosyl sulfuric acid, carry out diazotization reaction for 2 hours, and prepare diazonium salt solution.
[0030] (2) Dissolve 0.6035 g (4.4 mmol) of the coupling component N, N-dimethyl-3-aminophenol, 0.16 g of urea and 1.6 g of sodium acetate in 20 ml of methanol, cool to -10-5 ° C, and stir , slowly drop the above diazonium salt solution into the reaction system, continue to stir for 4 hours, let stand overnight, filter, wash with water, and dry to obtain the azo dye 2-[2-(5-methyl-1,3,4- Thiadiazolyl)azo]-5-(N,N-dimethylamino)phenol 0.705 g.
[0031] Yield: 67%, melting point: 229-230°C.
[0032] The characteristic absorption peaks of infrared spectrum (FT-IR) are: 3127, 2960, 2925, 2872, 1644, 1535, 1440, 1384, 13...
Embodiment 3
[0042] (1) Dissolve 0.5167 g (4.0 mmol) of the diazo component 2-amino-5-ethyl-1,3,4-thiadiazole in 4 ml of acetic acid and 2 ml of propionic acid, then stir, at -10-5 Under the condition of ℃, add 0.4ml sulfuric acid, slowly add 1.22 grams (4.8mmol) of 50% nitrosyl sulfuric acid, carry out diazotization reaction for 2 hours, and prepare diazonium salt solution.
[0043] (2) Dissolve 0.6035 g (4.4 mmol) of the coupling component N, N-dimethyl-3-aminophenol, 0.16 g of urea and 1.6 g of sodium acetate in 20 ml of methanol, cool to -10-5 ° C, and stir , slowly drop the above diazonium salt solution into the reaction system, continue to stir for 4 hours, let it stand overnight, filter, wash with water, and dry to obtain the azo dye 2-[2-(5-ethyl-1,3,4- Thiadiazolyl)azo]-5-(N,N-dimethylamino)phenol 0.798g. Yield: 72%.
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com