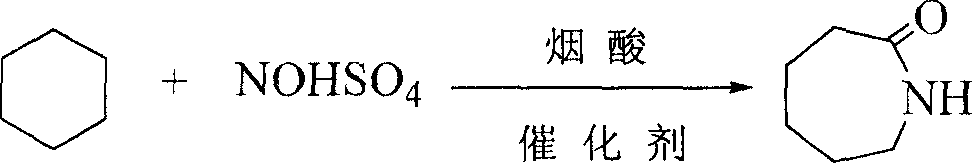Synthesis of caprolactam and its oligomer
A technology of caprolactam and oligomers, which is applied in the field of synthesis of caprolactam and its oligomers, can solve the problems of low-value by-products, low resource utilization, consumption of fuming sulfuric acid, etc., and achieve simple preparation methods, serious equipment corrosion, The effect of high power consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: by cyclohexane: nitrosyl sulfuric acid: nicotinic acid=10g: 4g: 4g material is packed in the 100mL glass reactor, reaction temperature 81 ℃, time 24h, the amount of catalyst accounts for cyclohexane 5%, the reacted mixture is divided into two layers, the upper layer is a colorless and transparent liquid which is cyclohexane, the lower layer is a viscous solid which is caprolactam, the remaining reactant nitrosylsulfuric acid, nicotinic acid and by-products. After liquid separation, the upper layer is weighed and analyzed by gas chromatography, and the lower layer is neutralized with barium hydroxide to remove SO 4 2- analyzed by liquid chromatography.
[0021] The different metal salts of table 1 are used as catalysts
[0022] catalyst
[0023] The generated oligomers are dimers and trimers of caprolactam, and the dimers and trimers of caprolactam are not included in the selectivity calculation of caprolactam.
Embodiment 2
[0024] Embodiment 2: cyclohexane: nitrosyl sulfuric acid: nicotinic acid=10g: 4g: 4g, the steps are the same as in Example 1, the difference is that 2g of glacial acetic acid is used as a catalyst, the reaction temperature is 81°C, and the time is 24h. The final mixture is divided into two layers, the upper layer is a colorless transparent liquid of cyclohexane, the lower layer of viscous solid is caprolactam, the remaining reactant nitrosylsulfuric acid and nicotinic acid and by-products. After liquid separation, the upper layer was weighed and analyzed by gas chromatography. Add a small amount of double distilled water to the solid in the lower layer in an ice bath, then slowly add concentrated ammonia water dropwise to neutralize to a pH of 8-9, then extract three times with an equal volume of ether, and use a certain amount of anhydrous Na 2 SO 4 Dry, add a certain amount of water or methanol to dissolve the residue after removing ether, and analyze it by liquid chromatog...
Embodiment 3
[0028] Embodiment 3: cyclohexane: nitrosyl sulfuric acid: nicotinic acid=10g: 4g: 4g, the steps are the same as in Example 1, the difference is that with 5% CuO / η-Al 2 o 3 It is a catalyst, the amount of the catalyst accounts for 1% of cyclohexane, the reaction temperature is 81 ° C, and the time is 24 hours. The mixture after the reaction is divided into two layers. The upper layer is a colorless transparent liquid. Reactants nitrosylsulfuric acid and nicotinic acid and by-products. After liquid separation, the upper layer is weighed and analyzed by gas chromatography, and the lower layer solid is processed by two methods:
[0029] 1. with embodiment 2; 2. with embodiment 1.
[0030] Cyclohexane conversion: 5.90%
[0031] Caprolactam selectivity: 0.67% (processing mode is the same as embodiment 2)
[0032] 3.16% (processing mode is the same as embodiment 1)
[0033] The generated oligomers are dimers and trimers of caprolactam, and the dimers and trimers ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com