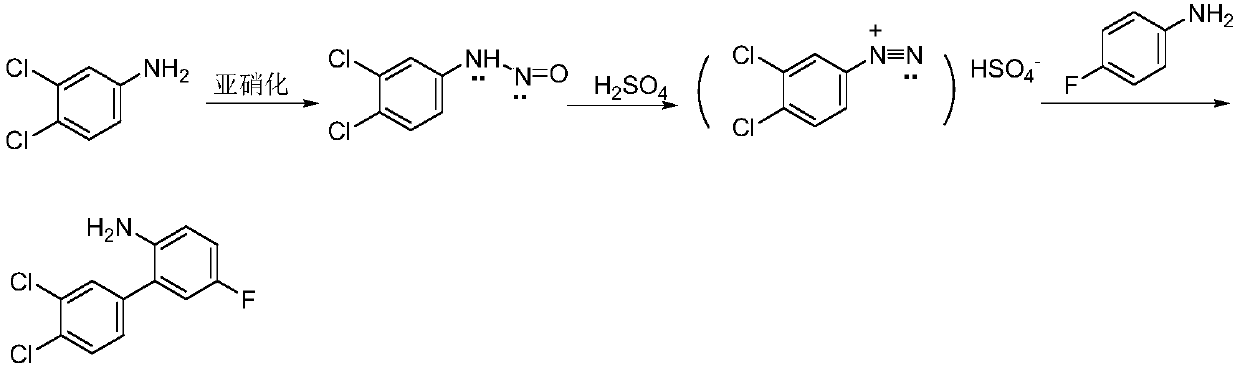
Method for synthesizing 3, 4-dichloro-2-amino-5-fluorobiphenyl
A technology of fluorobiphenyl and dichloroaniline, which is applied in the field of synthesizing 3,4-dichloro-2-amino-5-fluorobiphenyl, can solve the problems of inconvenient industrial operation, high price, complicated operation, etc., and achieve raw material The conversion rate and product selectivity are high, the effect of reducing industrial costs and improving economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Synthesis of diazonium salt
[0033] Add 32.4g of 3,4-dichloroaniline (200mmol), 33.0mL of concentrated sulfuric acid and 100mL of water in sequence to a 1000mL four-neck flask equipped with a stirrer, stir at 0°C for 15min, then add 60.6g of nitrous acid dropwise under nitrogen atmosphere 56% sulfuric acid solution (210mmol) of base sulfuric acid, continue to react for 30min after dripping, and filter out the insoluble matter while cold. Slowly add pre-cooled 200mL sodium hydroxide aqueous solution (4mol / L) to the filtrate, the pH is about 10, stir at low temperature for 10min to obtain the diazonium salt, which is directly used in the next reaction.
[0034] (2) Synthesis of 3,4-dichloro-2-amino-5-fluorobiphenyl
[0035] Add diazonium salt dropwise to 233.31g of 4-fluoroaniline (2.1mol) equipped with a stirrer and preheated to 90°C, the dropwise addition time is controlled within 30min, vigorously stirred for 45min, cooled, ethyl acetate (500mL× 3) extraction, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com