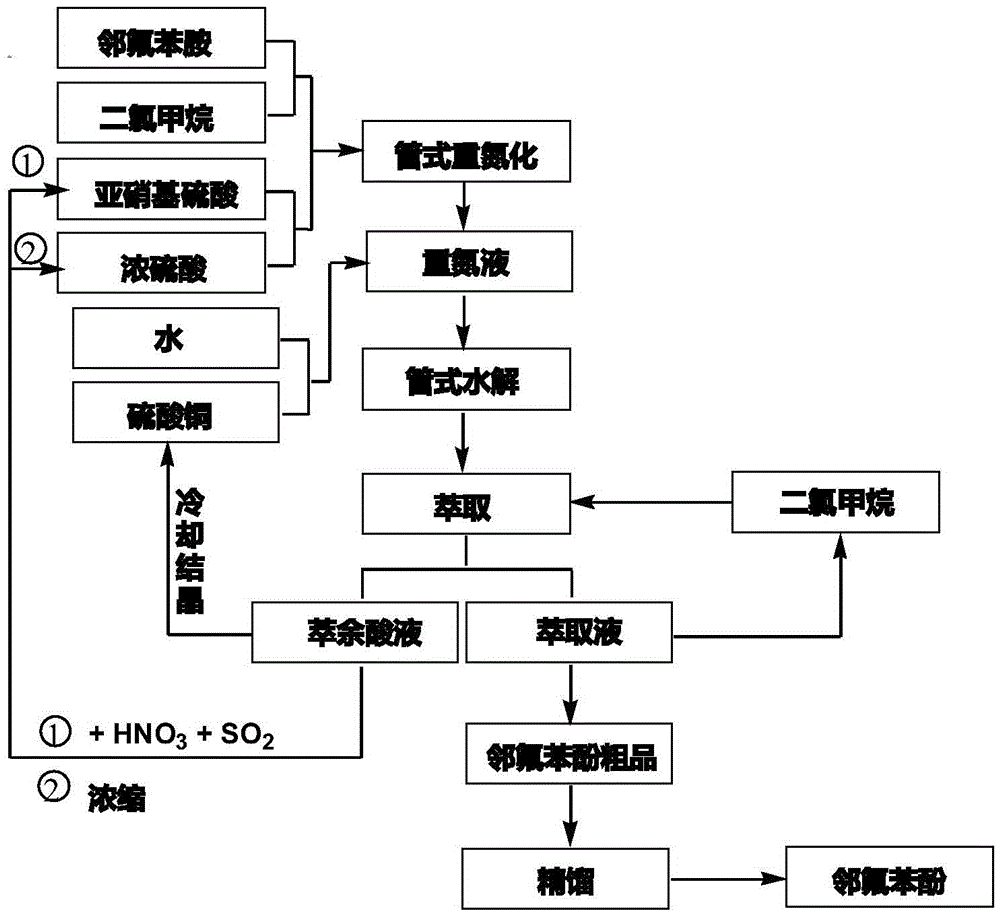
Tubular continuous o-fluorophenol production method
A technology of o-fluorophenol and o-fluoroaniline, which is applied in the field of tubular continuous production of o-fluorophenol, can solve the problems of reverse mixing of materials and uneven temperature distribution of materials, and improve the yield of diazonium reaction, increase yield, The effect of increasing product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Step (1): Tubular diazonium reaction: pump 220Kg o-fluoroaniline and 600Kg dichloromethane into the stirred tank, stir well and pump into the o-fluoroaniline high tank; then pump into the stirred tank
[0045] 660Kg of nitrosylsulfuric acid solution (the concentration of nitrosylsulfuric acid is 45.8wt%) and 355Kg of concentrated sulfuric acid are pumped into the nitrosylsulfuric acid header tank after stirring evenly. Open the bottom valves of the two high-level tanks, control the flow ratio to 1:1.24, mix evenly through the static mixer and enter the tubular diazonium reactor, the molar ratio of o-fluoroaniline and nitrosyl sulfuric acid is 1:1.2; temperature control At 20°C, the generated diazo solution enters the liquid storage tank.
[0046] Step (2): Tubular hydrolysis reaction: 550Kg copper sulfate pentahydrate and 1210Kg water are put into the reactor, stirred and clarified, then pumped into the copper sulfate solution high level tank; the diazo solution in the ...
Embodiment 2
[0049] Step (1): nitric acid was added to the remaining acid solution recovered in Example 1, and the mass fraction of nitric acid in the mixed solution obtained by stirring and mixing was 25.5wt%. Sulfur dioxide is absorbed in the falling film absorption tower connected in two series, and when the concentration of nitrosyl sulfuric acid in the obtained nitrosyl sulfuric acid solution A is 30wt%, the nitrosyl sulfuric acid solution A is produced.
[0050] Step (2): Tubular diazonium reaction: add a dichloromethane solution of o-fluoroaniline (220Kg) into the stirred tank, stir evenly and pump it into the o-fluoroaniline-dichloromethane high level tank; then pump it into the stirred tank The nitrosylsulfuric acid solution A1000kg that step (1) makes is pumped into the nitrosylsulfuric acid head tank after stirring evenly. Open the bottom valves of the two high level tanks, and control the molar ratio of o-fluoroaniline and nitrosyl sulfuric acid to be 1:1.2. After the static m...
Embodiment 3
[0055] Step (1): Tubular diazonium reaction: 44.4g o-fluoroaniline and 240g dichloromethane are mixed and stirred (o-fluoroaniline solution); 132g nitrosylsulfuric acid (46.16wt%) is mixed and stirred with 66g vitriol oil ( nitrosylsulfuric acid solution). The o-fluoroaniline solution and the nitrosyl sulfuric acid solution were pumped into the silica gel tube by a peristaltic pump to carry out the diazotization reaction. The silica gel tube was placed in a water bath at 20° C., and the residence time in the tube was 15 s.
[0056] Step (2): Tubular hydrolysis reaction: Mix 100g of copper sulfate pentahydrate and 200g of water to form a clarified liquid and a diazo liquid (maintain the liquid temperature at 20°C). The peristaltic pump is pumped into the silicone tube for tubular hydrolysis reaction. The tube length is 20 m. The silicone tube is placed in an oil bath at 135° C., and the residence time in the tube is 20 min to obtain a hydrolyzate.
[0057] Step (3): Extraction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com