Method for continuously producing resorcin
A technology of resorcinol and m-phenylenediamine, which is applied in the field of continuous production of resorcinol in a loop reaction device, can solve the problems of high control requirements of production equipment and operation process, small production capacity per unit volume of equipment and poor product quality. Stability and other problems, to achieve the effect of solving the uneven temperature distribution of the material, solving the back mixing of the material, and being easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
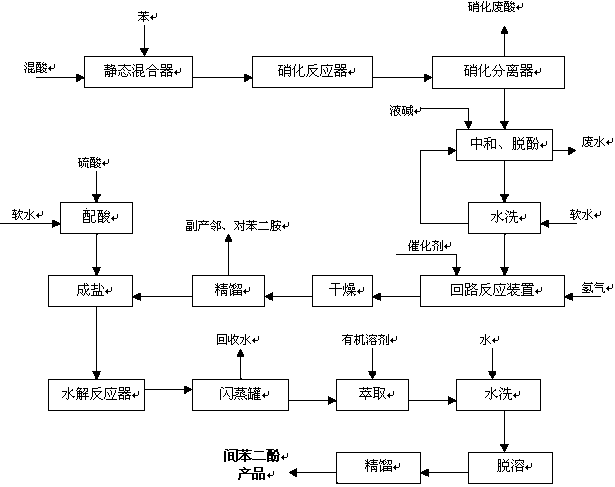
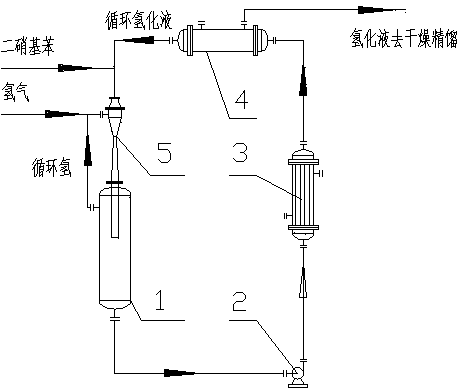
[0028] Embodiment 1: as figure 1 Shown to produce resorcinol
[0029] (1) Preparation of dinitrobenzene by nitration of benzene: The mixed acid containing 70% sulfuric acid, 10% nitric acid, and 20% water is continuously sent to the static mixer together with benzene, and then enters the nitration microchannel reactor for adiabatic nitration reaction , the molar feeding ratio of nitric acid and benzene is 2:1.05, the nitrification temperature is controlled at 120°C, the residence time of the reaction material is 1s, the nitrification liquid enters the separator continuously, and the waste acid is separated to obtain crude dinitrobenzene, and the waste acid is dehydrated by flash evaporation Remove the waste acid storage tank, then use fresh benzene to extract and treat it, and then denitrify the reactor for recycling. The crude product of dinitrobenzene is neutralized, dephenolized, washed and refined to obtain dinitrobenzene, and the yield of dinitrobenzene is 99.1 %, the pu...
Embodiment 2
[0032] Embodiment 2: as figure 1 Shown to produce resorcinol
[0033] (1) Preparation of dinitrobenzene by nitration of benzene: (1) Preparation of dinitrobenzene by nitration of benzene: The mixed acid containing 77% sulfuric acid, 8% nitric acid and 15% water is continuously fed into the static mixer together with benzene, Then enter the nitration microchannel reactor for adiabatic nitration reaction, the molar feeding ratio of nitric acid and benzene is 2:1.13, the nitration temperature is controlled at 150°C, the residence time of the reaction material is 10s, the nitration liquid enters the separator continuously, and the spent acid is separated to obtain The crude product of dinitrobenzene, the waste acid is flashed and dehydrated to the waste acid storage tank, and then extracted with fresh benzene, then the denitration reactor is recycled, and the crude product of dinitrobenzene is neutralized, dephenolized, washed and refined to obtain dinitrobenzene The yield of nit...
Embodiment 3
[0036] Embodiment 3: as figure 1 Shown to produce resorcinol
[0037] (1) Preparation of dinitrobenzene by nitration of benzene: (1) Preparation of dinitrobenzene by nitration of benzene: The mixed acid containing 85% sulfuric acid, 10% nitric acid and 5% water is continuously fed into the static mixer together with benzene, Then enter the nitration microchannel reactor for adiabatic nitration reaction, the molar feeding ratio of nitric acid and benzene is 2:1.2, the nitration temperature is controlled at 180°C, the residence time of the reaction material is 20s, the nitration liquid enters the separator continuously, and the waste acid is separated to obtain The crude product of dinitrobenzene, the waste acid is flashed and dehydrated to the waste acid storage tank, and then extracted with fresh benzene, then the denitration reactor is recycled, and the crude product of dinitrobenzene is neutralized, dephenolized, washed and refined to obtain dinitrobenzene The yield of nitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com