Single diazo compound, its preparation method and use
A compound and monoazo technology, applied in the field of monoazo compounds, can solve problems such as the inability to meet environmental protection requirements, and achieve the effect of good color fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] ① Diazotization
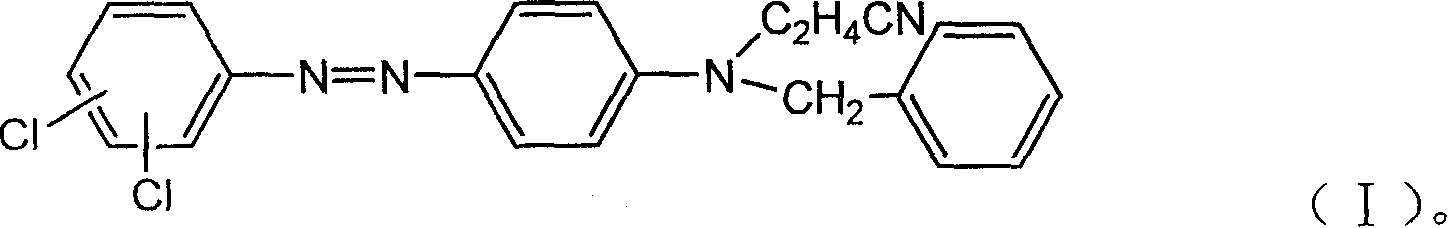
[0019] Slowly add sulfuric acid and nitrosylsulfuric acid into a 150ml three-neck flask, and cool down with a water bath outside to control the temperature at about 15-20°C. After stirring for 0.5hr, add 20 grams of 3,4-dichloroaniline. The addition should not be too fast. A certain feeding speed should be controlled to prevent the material from agglomerating. The entire feeding time is controlled at 1.5hr. End point (1. Diazonium salt can be clear and transparent in ice water; 2. Diazonium salt can make starch potassium iodide test paper turn blue), and the prepared 3,4-dichloroaniline diazonium salt is set aside.
[0020] ②Coupling reaction
[0021] Add 200ml of absolute alcohol to a 1000ml beaker, add 28 grams of N-cyanoethyl-N-benzylaniline, 1.2 grams of emulsifier TX-15, beat for 3 hours, add 1.5 grams of sulfamic acid, and control the temperature at about 20 °C Add diazonium solution dropwise for 1.5 hr, stir and react at 20°C for 12 hr, measur...
Embodiment 2
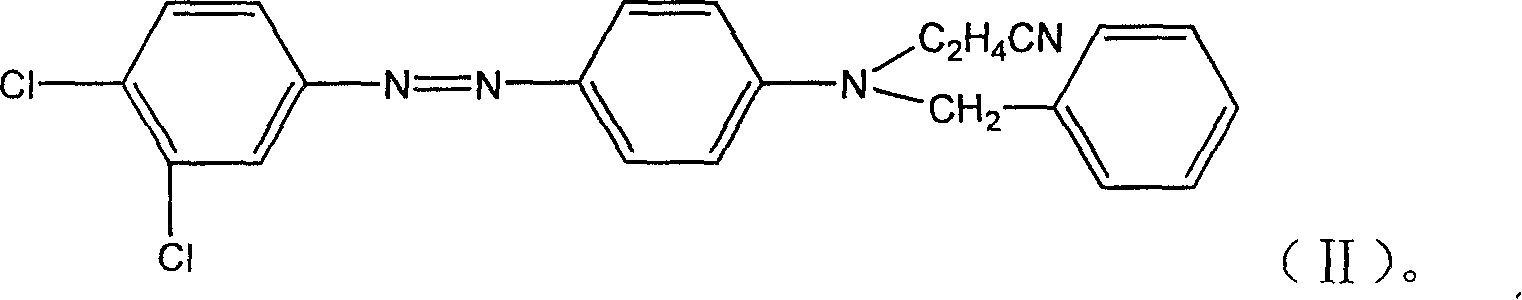
[0026] Add 200ml of DMF to a 1000ml beaker, add 28 grams of N-cyanoethyl-N-benzylaniline, stir to dissolve it, add 1.5 grams of sulfamic acid, control the temperature at about 20 °C and add dropwise Example 1 to obtain heavy Nitrogen solution, the drop time is 1.5hr, stir and react at 20°C for 12hr, measure the reaction end point (use the osmosis ring method to check that the diazonium salt is slightly excessive), continue to keep warm and stir for 40 minutes, filter and wash with water until neutral, and get a paste The substance is the product.
[0027] Grinding the obtained paste with the diffusing agent CNF at a ratio of 1:2, and then spray drying to obtain the disperse yellow dye of the present invention.
[0028] Test the color fastnesses of disperse yellow dyes according to the method described in Example 1, and the test results are shown in Table 1.
Embodiment 3
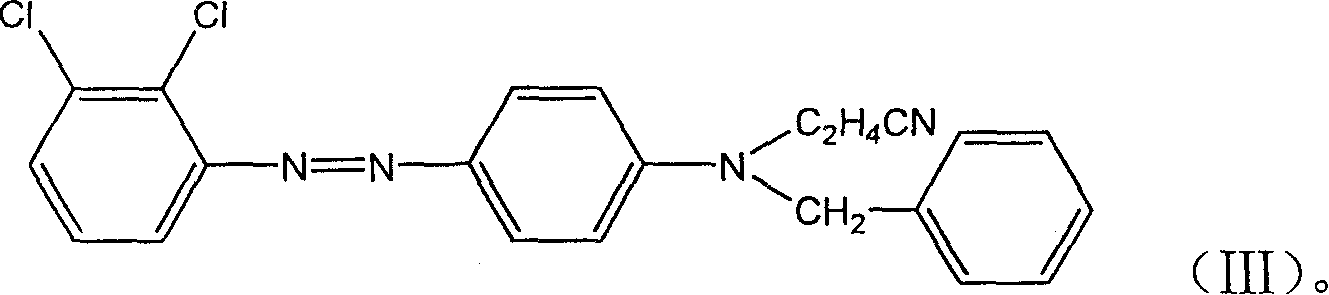
[0030] Substitute 3,4-dichloroaniline with 2,3-dichloroaniline, and prepare according to the method of Example 1 to obtain the disperse yellow dye of the present invention.
[0031] Test the color fastnesses of disperse yellow dyes according to the method described in Example 1, and the test results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com