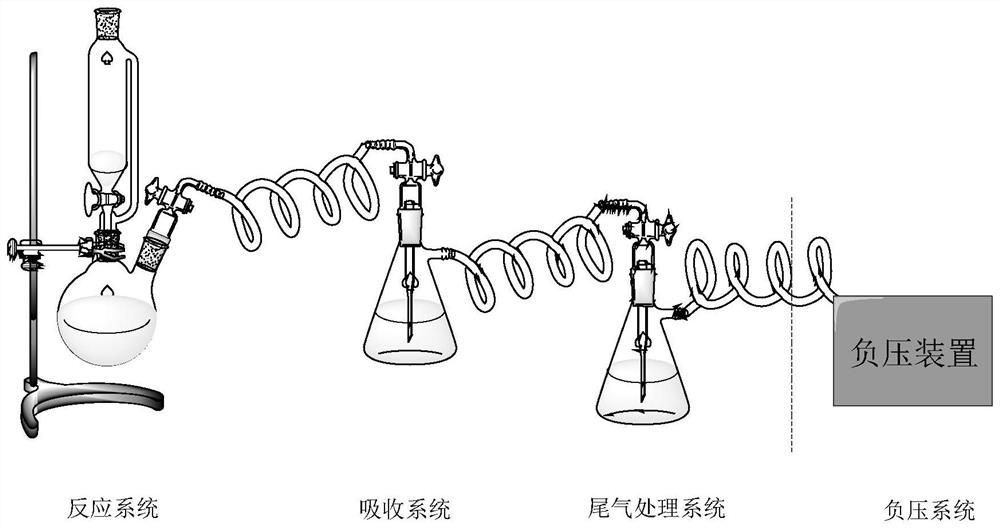
Recycling method of nitrogen oxide gas in diazotization reaction process
A reaction process and nitrogen oxide technology, applied in chemical instruments and methods, separation methods, organic chemistry, etc., can solve problems such as the inability to realize waste gas recycling, and achieve the effect of solving environmental problems and improving economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
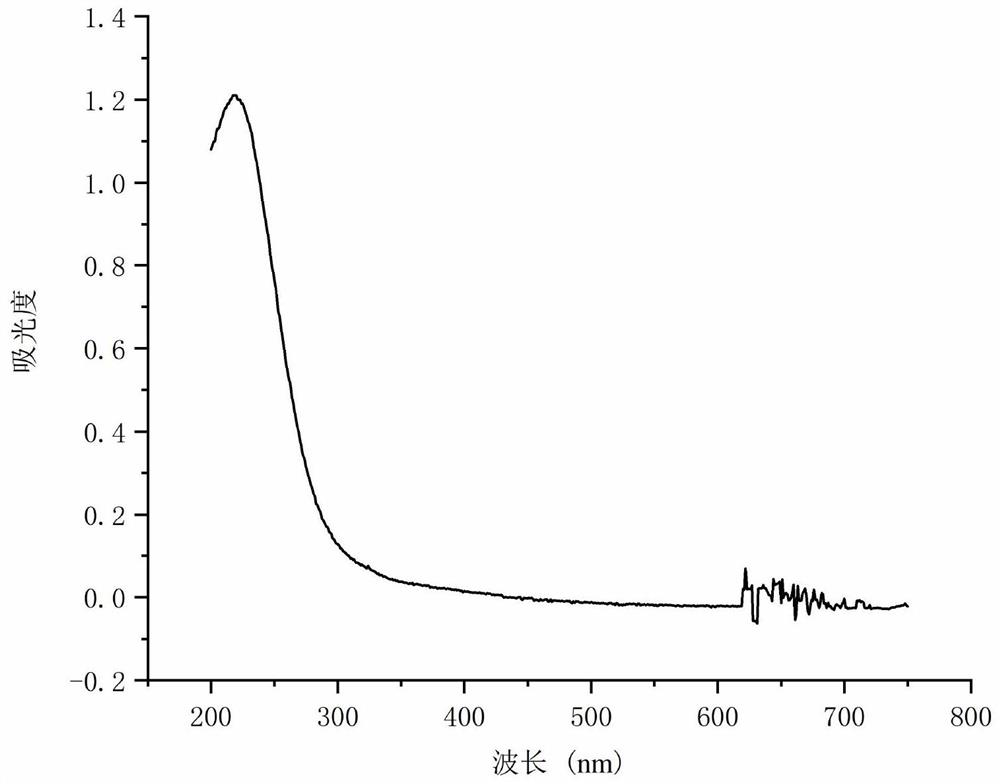
[0027] (1) NO X Gas absorption: Add 88 mL of 69 wt% sulfuric acid to a 250 mL two-necked flask, then add 11.8 g of m-nitroaniline, and stir to form a suspension; slowly dissolve 23 g of 36 wt% sodium nitrite solution Add dropwise to the two-necked flask. The NO produced during the dropping process X The gas is passed into an absorption bottle filled with 5 mL of concentrated sulfuric acid using a negative pressure device. The temperature of concentrated sulfuric acid was kept at 20°C. reabsorbed NO X Gas five times until all the concentrated sulfuric acid in the absorption bottle turns into solid. Heat the solid in the absorption bottle to dissolve, then take 10 μL of the sample and dissolve it in a small amount of concentrated sulfuric acid, transfer it to a 50 mL volumetric flask, continue diluting with concentrated sulfuric acid, and constant volume, the concentration is 222 mg measured by UV-Vis spectrometer / L, its ultraviolet characteristic peaks are as figure 1 . ...
Embodiment 2
[0031] (1) NO X Gas absorption: Add 88 mL of 69 wt% sulfuric acid to a 250 mL two-necked flask, then add 13.9 g of 3,5-dichloroaniline, and stir to form a suspension; 23 g of 36 wt% sulfuric acid The sodium nitrate solution was slowly added dropwise into the two-necked flask. The NO produced during the dropping process X The gas is passed into an absorption bottle filled with 5 mL of concentrated sulfuric acid using a negative pressure device. The temperature of concentrated sulfuric acid was kept at 30°C. reabsorbed NO X Gas five times until all the concentrated sulfuric acid in the absorption bottle turns into solid. The solid in the absorption bottle was heated and dissolved, and the sample was prepared, and the concentration was measured as 246 mg / L by an ultraviolet-visible spectrometer. After calculation, the mass fraction of nitrosyl sulfuric acid in the measured absorption liquid is 76%.
[0032] (2) Reuse of absorption liquid: add 22 mL of 69 wt% sulfuric acid i...
Embodiment 3
[0035] (1) NO X Gas absorption: Add 88 mL of 69 wt% sulfuric acid to a 250 mL two-necked flask, then add 13.0 g of 2-methyl-4-nitroaniline, and stir to form a suspension; 23 g of 36 wt% % sodium nitrite solution was slowly dropped into the two-necked flask. The NO produced during the dropping process X The gas is passed into an absorption bottle filled with 5 mL of concentrated sulfuric acid using a negative pressure device. The temperature of the concentrated sulfuric acid is maintained at 50°C. reabsorbed NO X Gas seven times, so that the concentrated sulfuric acid in the absorption bottle reaches the maximum adsorption capacity, take an appropriate amount of the absorption solution for sample preparation, and use the ultraviolet-visible spectrometer to measure its concentration as 309 mg / L. After calculation, the mass fraction of nitrosyl sulfuric acid in the measured absorption liquid was 96%.
[0036] (2) Reuse of absorption solution: Add 22 mL of 69 wt% sulfuric aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com