Process for preparing nitroso sulfuric acid by recycling cyclopropylamine to produce tail gas sulfur dioxide
A technology of nitrosyl sulfuric acid and sulfur dioxide, which is applied in the direction of inorganic chemistry, nitrogen compounds, chemical instruments and methods, etc., can solve the problems of increasing environmental protection costs, decomposition, cumbersome process, etc., and achieve the goals of reducing decomposition, ensuring quality, and reducing reaction temperature Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
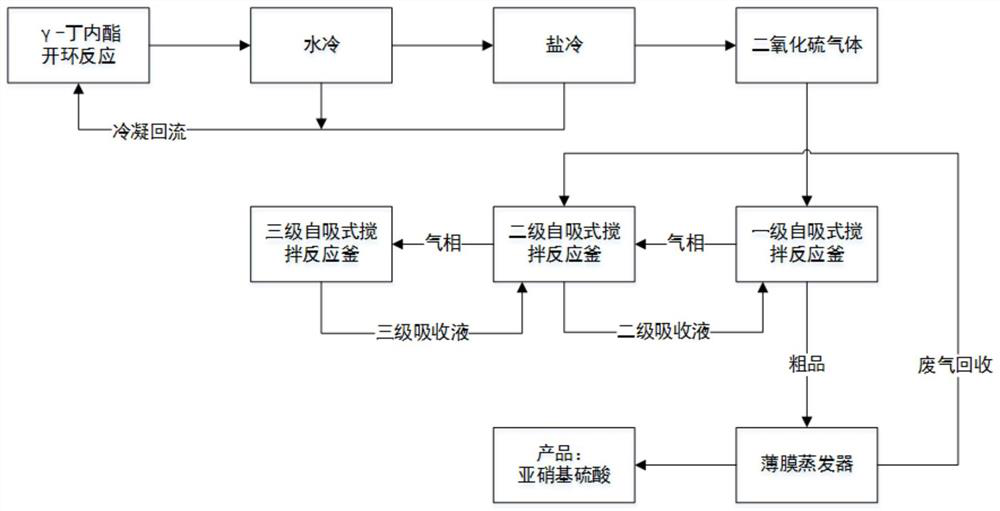
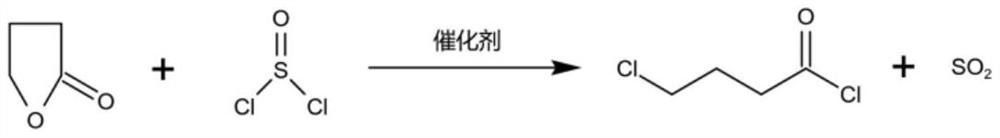
[0059] (1) 860kg of γ-butyrolactone and 45kg of catalyst zinc chloride are dropped into the ring-opening reaction kettle, 1780kg of thionyl chloride is added dropwise, the reaction temperature is controlled at 50°C, and the reaction time is 20h, and the gas generated by the reaction passes through the water cooler (20°C). °C) and a salt cooler (-15 °C), the condensate is returned to the open-loop reactor to obtain by-product sulfur dioxide gas;
[0060] (2) The sulfur dioxide gas is passed into the first-level absorption kettle, and the mixed acid mass ratio in the first-level absorption kettle is 88% sulfuric acid 1000kg, 97% nitric acid 320kg, 40% nitroso sulfuric acid 50kg, and the temperature of the first-level absorption kettle is controlled to be 20~25 ℃, the inlet pressure of the first-stage absorption kettle is adjusted to 10kpa. The mixed acid mass ratio in the secondary absorption kettle is 88% sulfuric acid 1000kg, 97% nitric acid 320kg, 40% nitroso sulfuric acid 50...
Embodiment 2
[0065] (1) 860kg of γ-butyrolactone and 70kg of catalyst zinc chloride are dropped into the ring-opening reaction kettle, 1550kg of thionyl chloride is added dropwise, the reaction temperature is controlled at 55°C, and the reaction time is 15h, and the gas generated by the reaction passes through the water cooler (25°C). °C) and a salt cooler (-13 °C), the condensate is returned to the open-loop reactor to obtain by-product sulfur dioxide gas;
[0066] (2) sulfur dioxide gas is passed into the first-level absorption kettle, and the mixed acid mass ratio in the first-level absorption kettle is 80% sulfuric acid 975kg, 97% nitric acid 315kg, 60% nitroso sulfuric acid 40kg, and the first-level absorption kettle temperature is controlled to be 25~30 ℃, the inlet pressure of the first-stage absorption kettle is adjusted to 10kpa. The mixed acid mass ratio in the secondary absorption kettle is 80% sulfuric acid 975kg, 97% nitric acid 315kg, 60% nitroso sulfuric acid 40kg, the unabs...
Embodiment 3
[0071] (1) drop into γ-butyrolactone 860kg and catalyst zinc chloride 60kg in the open-loop reaction kettle, drip 1650kg of thionyl chloride, temperature of reaction is controlled at 60 ℃, reaction time 18h, the gas that the reaction produces passes through water cooler (15 °C) and a salt cooler (-20 °C), the condensate is returned to the open-loop reactor to obtain by-product sulfur dioxide gas.
[0072] (2) The sulfur dioxide gas is passed into the first-level absorption kettle, and the mixed acid mass ratio in the first-level absorption kettle is 920kg of 90% sulfuric acid, 325kg of 97% nitric acid, 55kg of 50% nitroso sulfuric acid, and the temperature of the first-level absorption kettle is controlled to be 18~23 ℃, the inlet pressure of the first-stage absorption kettle is adjusted to 10kpa. The mixed acid mass ratio in the secondary absorption kettle is 920kg of 90% sulfuric acid, 325kg of 97% nitric acid, 55kg of 50% nitroso sulfuric acid, and the unabsorbed tail gas f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com