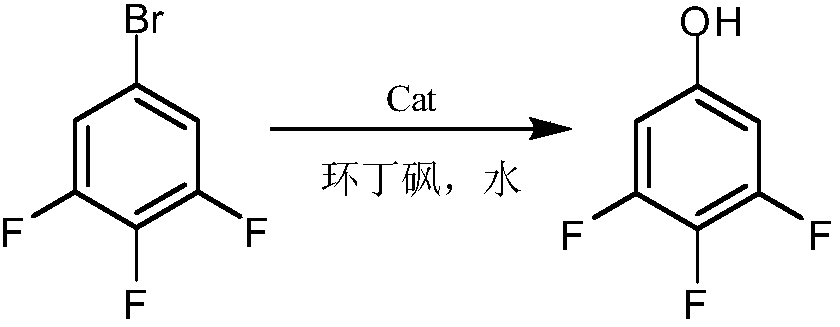
Synthesis method of 3, 4, 5-trifluorophenol
A technology of trifluorophenol and synthesis method, which is applied in the preparation of liquid crystal material intermediates, pesticides, and pharmaceutical fields. It can solve the problems of ammoniation and hydrolysis catalysts, high safety risks, and environmental problems, and reduce by-products and tar. The effect of avoiding the generation of high-salt wastewater and reducing the risk of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
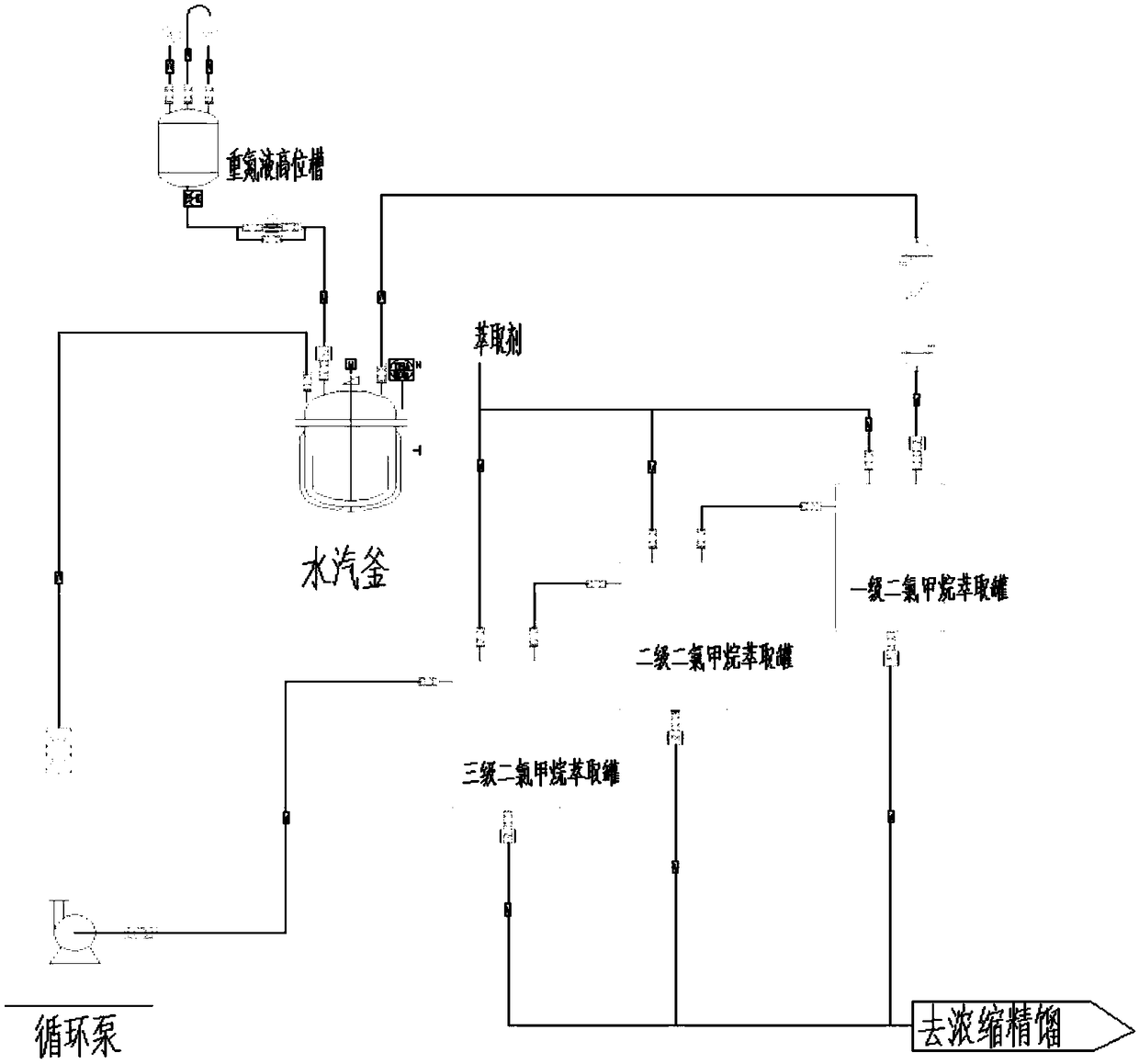
[0039] (1) Add 160g 3,4,5-trifluorobromobenzene and 6.4g Cu to a 1000mL autoclave 2 O-bipyridine catalyst, measure 480mL ammonia water (ammonia water concentration is 25%), add it to the autoclave, seal the autoclave, stir and heat up to 160℃, stirring speed 500r / min, reaction for 10h, the highest pressure during the reaction 1.5Mpa; Open the autoclave when it is lowered to room temperature, transfer the materials in the kettle for vapor distillation, the distilled materials are cooled to obtain a white solid, filtered and dried to obtain a white product of about 106.4g with a purity of 99.6% and a molar yield 95.1%;
[0040] (2) Add 300g of 98% concentrated sulfuric acid, 100g of water, and 147g of 3,4,5-trifluoroaniline to a 100mL three-necked flask, stir, heat up to 70℃ to dissolve, keep for 30min, cool to -5~0℃, drop Add 350 g of 40% nitrosyl sulfuric acid solution, drip and keep for 1 hour to obtain diazonium liquid for use;
[0041] (3) such as figure 1 As shown, add 450g o...
Embodiment 2
[0044] (1) Add 160g 3,4,5-trifluorobromobenzene and 6.4g Cu to a 1000mL autoclave 2 O-bipyridine catalyst, measure 480mL ammonia water (ammonia water concentration is 25%), add it to the autoclave, seal the autoclave, stir and heat up to 150℃, stirring speed 500r / min, reaction for 16h, the highest pressure during the reaction 1.3MPa; Open the autoclave when it drops to room temperature, transfer the materials in the kettle for vapor distillation, and obtain a white solid after cooling the distillate, which is filtered and dried to obtain a white product of about 103.2g with a purity of 99.6% and a molar yield 92.4%.
[0045] (2) 1000mL three-necked flask, add 300g concentrated sulfuric acid, 100g water, 147g 3,4,5-trifluoroaniline, stir, heat up to 70℃ to dissolve, keep for 30min, cool down to 0~5℃, add 40% nitrous acid dropwise 350g sulphuric acid, after dripping and holding for 1 hour, obtain diazonium liquid for use.
[0046] (3) such as figure 1 As shown, add 450g of sulfuric...
Embodiment 3
[0049] (1) Add 160g 3,4,5-trifluorobromobenzene and 6.4g Cu to a 1000mL autoclave 2 O-bipyridine catalyst, measure 400mL ammonia water (ammonia water concentration is 25%), add it to the autoclave, seal the autoclave, heat up to 160℃ with stirring, stir at 500r / min, react for 10h, the maximum pressure during the reaction 1.5MPa; Open the autoclave when it drops to room temperature, transfer the materials in the kettle for vapor distillation, and obtain a white solid after cooling the distillate, which is filtered and dried to obtain a white product of about 101.2g with a purity of 99.6% and a molar yield 90.6%.
[0050] (2) A 1000mL three-necked flask, add 300g of concentrated sulfuric acid, 100g of water, 147g of 3,4,5-trifluoroaniline, stir, heat to 70℃ to dissolve, keep for 30min, cool to -5~0℃, add 40% sub Nitrosulfuric acid 334g, after dripping and holding for 1h, the diazonium liquid is obtained for use.
[0051] (3) such as figure 1 As shown, add 450g of sulfuric acid solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com