Preparation method of p-chlorophenylhydrazine sulfate
A technology of p-chlorophenylhydrazine and sulfate, which is applied in the field of preparation of p-chlorophenylhydrazine sulfate, can solve the problems of high catalyst cost, multi-salt wastewater, complicated three-stage suction filtration process, etc., and achieve the effect of less three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
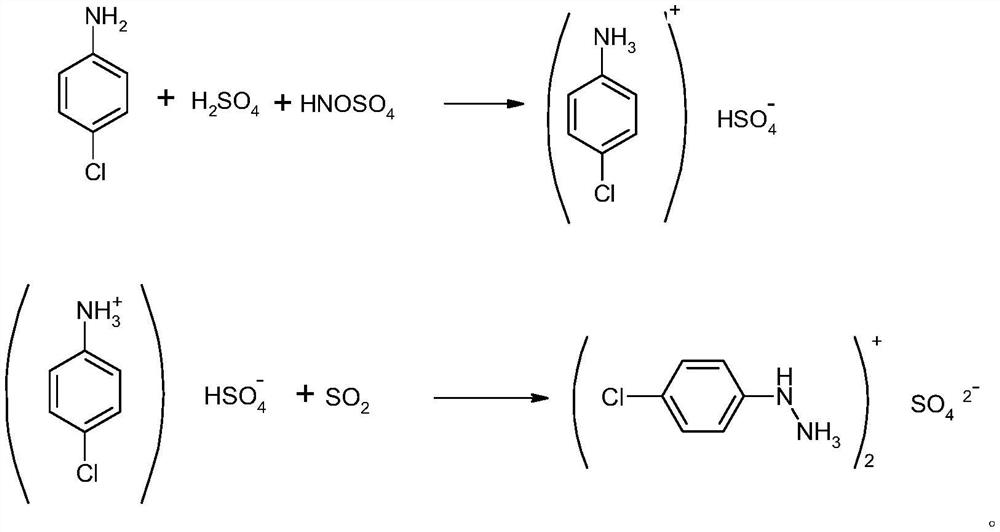
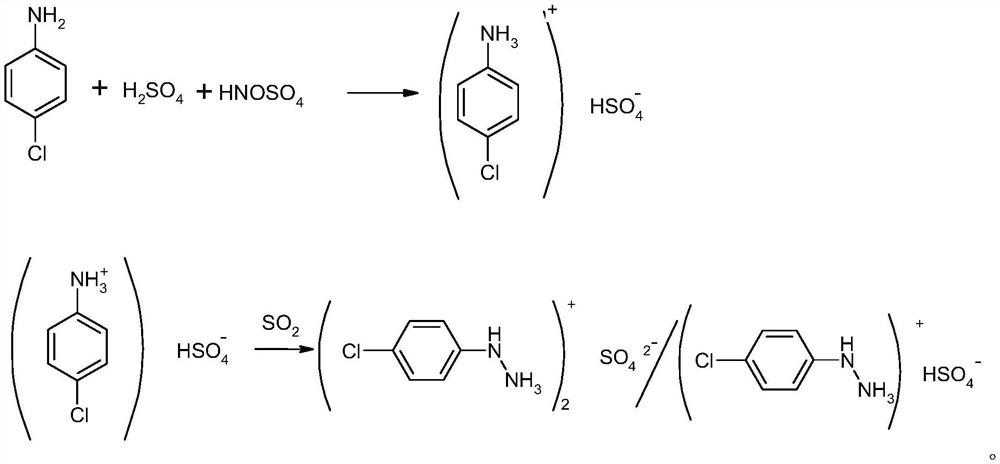
[0027] A preparation method of p-chlorophenylhydrazine sulfate: put 3075KG of 50% sulfuric acid into a 1000L reactor equipped with stirring, heat up to 65°C, add 1000KG of p-chloroaniline, and react for 2hr after adding, cool to 3°C, add dropwise 1195KG of 100% nitrosylsulfuric acid, the temperature was raised to 35°C after 2 hours of reaction after the dropwise addition, and kept for 2 hours, and 5270KG of diazo solution was obtained after passing through the filter press tank; the normalization of diazo solution was greater than 99%.
[0028] Put the diazo solution into the reduction autoclave, keep the reaction temperature at 20°C, pour in sulfur dioxide to 0.5MPa, keep the temperature and pressure to react for 6 hours, and keep feeding sulfur dioxide in the process, and a total of about 1305KG of sulfur dioxide is fed in; Reduction of intermediates>99%, after the reaction is qualified, release the pressure and raise the temperature to reflux for 1hr, take a sample and contr...
Embodiment 2
[0030] A preparation method of p-chlorophenylhydrazine sulfate: put 5125KG of 30% sulfuric acid into a 1000L reactor equipped with stirring, heat up to 80°C, add 1000KG of p-chloroaniline, and react for 2hr after adding, cool down to 0°C, add dropwise 1494KG of 80% nitrosyl sulfuric acid, the temperature is raised to 35°C after 1 hour of reaction after the dropwise addition, and the temperature is kept for 0.5 hours. After passing through the filter press tank, 7619KG of diazo solution is obtained; the normalization of diazo solution is greater than 99%.
[0031] Put the diazo solution into the reduction autoclave, keep the reaction temperature at 0°C, pour in sulfur dioxide to 0.5MPa, keep the temperature and pressure to react for 8 hours, and keep feeding sulfur dioxide in the process, and a total of about 1250KG of sulfur dioxide is fed in; Reduction of intermediates>99%, after the reaction is qualified, release the pressure and raise the temperature to reflux for 1hr, take ...
Embodiment 3
[0033] A preparation method of p-chlorophenylhydrazine sulfate: put 5125KG of 30% sulfuric acid into a 1000L reactor equipped with stirring, heat up to 80°C, add 1000KG of p-chloroaniline, and react for 2hr after adding, cool down to 0°C, add dropwise 1195KG of 100% nitrosyl sulfuric acid, the temperature was raised to 35°C after 1 hour of reaction after the dropwise addition, and kept for 1 hour, and 7320KG of diazo solution was obtained after passing through the filter press tank; the normalization of diazo solution was greater than 99%.
[0034] Put the diazo solution into the reduction autoclave, keep the reaction temperature at -5°C, pour in sulfur dioxide to 0.8MPa, keep the temperature and pressure to react for 10 hours, and keep feeding sulfur dioxide in the process, and a total of about 1280KG of sulfur dioxide is fed in; sampling is controlled in the reaction The reduced intermediate of the reaction is >99%, after the reaction is qualified, the pressure is released an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com