D-pi-A structure nonlinear optical chromophore, and its synthesizing method
A technology of nonlinear light and synthesis method, which is applied in the field of nonlinear optical chromophore and its synthesis, which can solve the problems such as the decrease of molecular transparency, and achieve the effects of high yield, high nonlinear optical performance and good transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
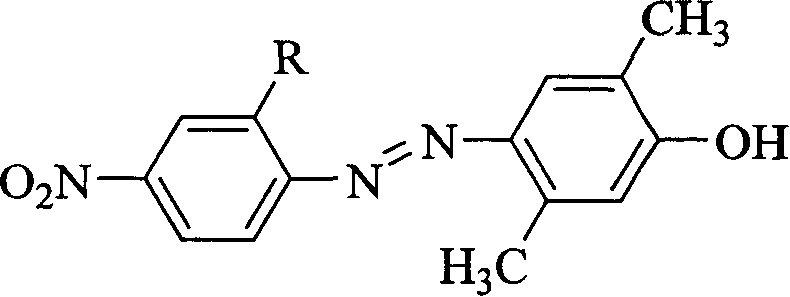
[0019] R is a chlorine atom, and the synthetic route of the nonlinear optical chromophore 2,5-dimethyl-4-(2'-chloro-4'-nitroazo)phenol is as follows:
[0020]
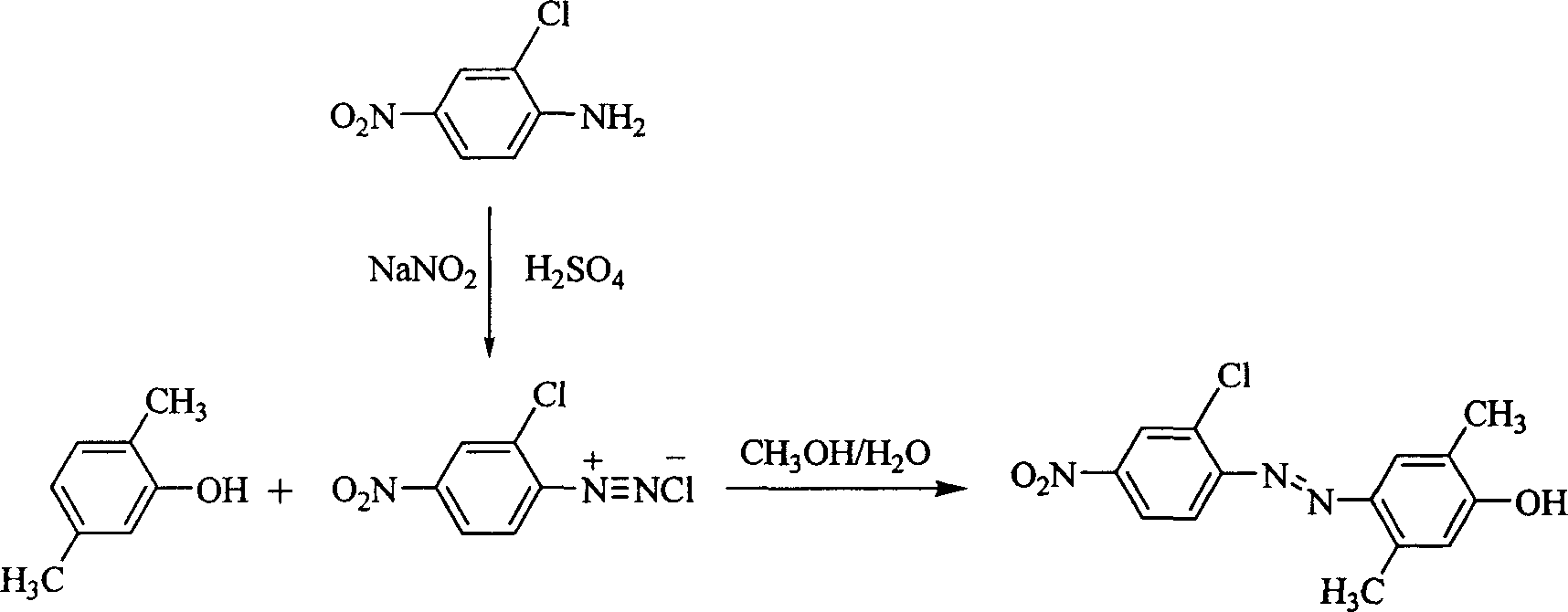
[0021] The synthesis method is as follows: 1.38g of sodium nitrite is slowly added in batches to 20ml of 98% concentrated sulfuric acid, the temperature is controlled below 20°C, stirred to dissolve completely, and then cooled to 0°C to obtain nitrosyl sulfuric acid. Then add 10ml of concentrated sulfuric acid to 3.45g (20mmol) of 2-chloro-4-nitroaniline, stir to form a paste, slowly add 20mmol of nitrosylsulfuric acid to the inside, and react at 0°C for 1 hour, then add 2.44g (20mmol) a mixed solution of 2,5-dimethylphenol and 10ml of methanol and water (the volume ratio of methanol to water is 2 / 1), react at 0°C for 1 hour, adjust the pH value to 7-8 with ammonia water and continue the reaction 2 hours. After standing overnight, filter with suction and wash with water to obtain the initial product. Then recrysta...
Embodiment 2
[0027] R is a chlorine atom, and the synthetic method of chromophore 2,5-dimethyl-4-(2'-chloro-4'-nitroazo)phenol: 1.38g sodium nitrite is slowly added to 20ml98 in batches % concentrated sulfuric acid, the temperature is controlled below 20°C, stirred to dissolve it completely, and then cooled to 0°C to obtain nitrosyl sulfuric acid. Then add 2ml of glacial acetic acid to 3.45g (20mmol) of 2-chloro-4-nitroaniline, stir to form a paste, slowly add 20mmol of nitrosylsulfuric acid inwardly, and react at 0°C for 1 hour, then add 3.66 g (30mmol) 2,5-xylenol and 100ml of methanol and water (the volume ratio of methanol to water is 2 / 1) mixed solution, reacted at 0°C for 1 hour, and adjusted the pH value to 7 with sodium carbonate solution -8 Continue to react for 2 hours. After standing overnight, filter with suction and wash with water to obtain the initial product. The mixed solvent of water and acetone is recrystallized twice to obtain the desired nonlinear optical chromophore...
Embodiment 3
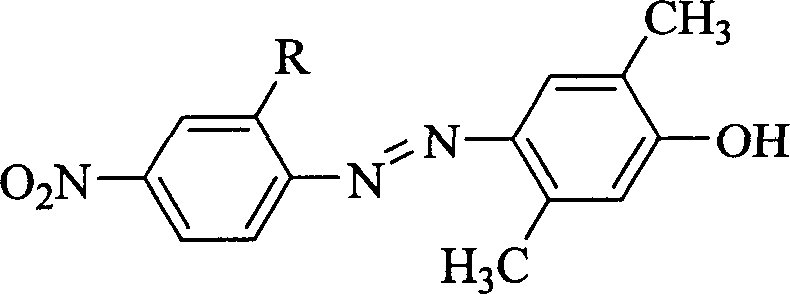
[0029] R is a bromine atom, and the synthetic route of the nonlinear optical chromophore 2,5-dimethyl-4-(2'-bromo-4'-nitroazo)phenol is as follows:
[0030]
[0031] The synthesis method is as follows: 0.14g of sodium nitrite is slowly added in batches to 2ml of 98% concentrated sulfuric acid, the temperature is controlled below 20°C, stirred to dissolve completely, and then cooled to 0°C to obtain nitrosyl sulfuric acid. Add 10ml of concentrated hydrochloric acid to 0.44g (2mmol) of 2-bromo-4-nitroaniline, stir to form a paste, slowly add 2mmol of nitrosylsulfuric acid to the inside, react at 0°C for 1 hour, then add 0.24g ( 2mmol) a mixed solution of 2,5-dimethylphenol and 6ml of methanol and water (the volume ratio of methanol to water is 2 / 1), react at 0°C for 1 hour, and adjust the pH value to 7-8 with sodium hydroxide solution The reaction was continued for 2 hours. After standing overnight, filter with suction and wash with water to obtain the initial product. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com