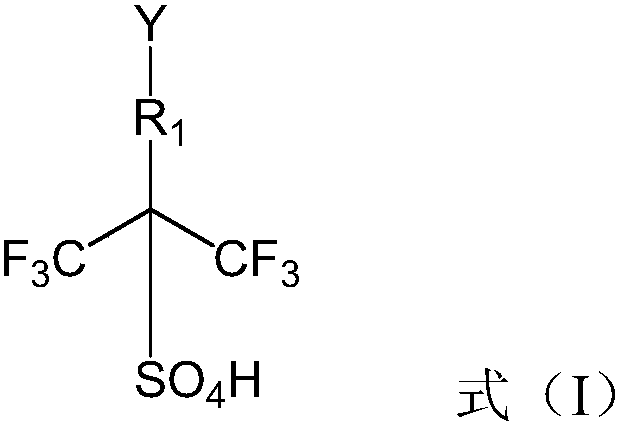
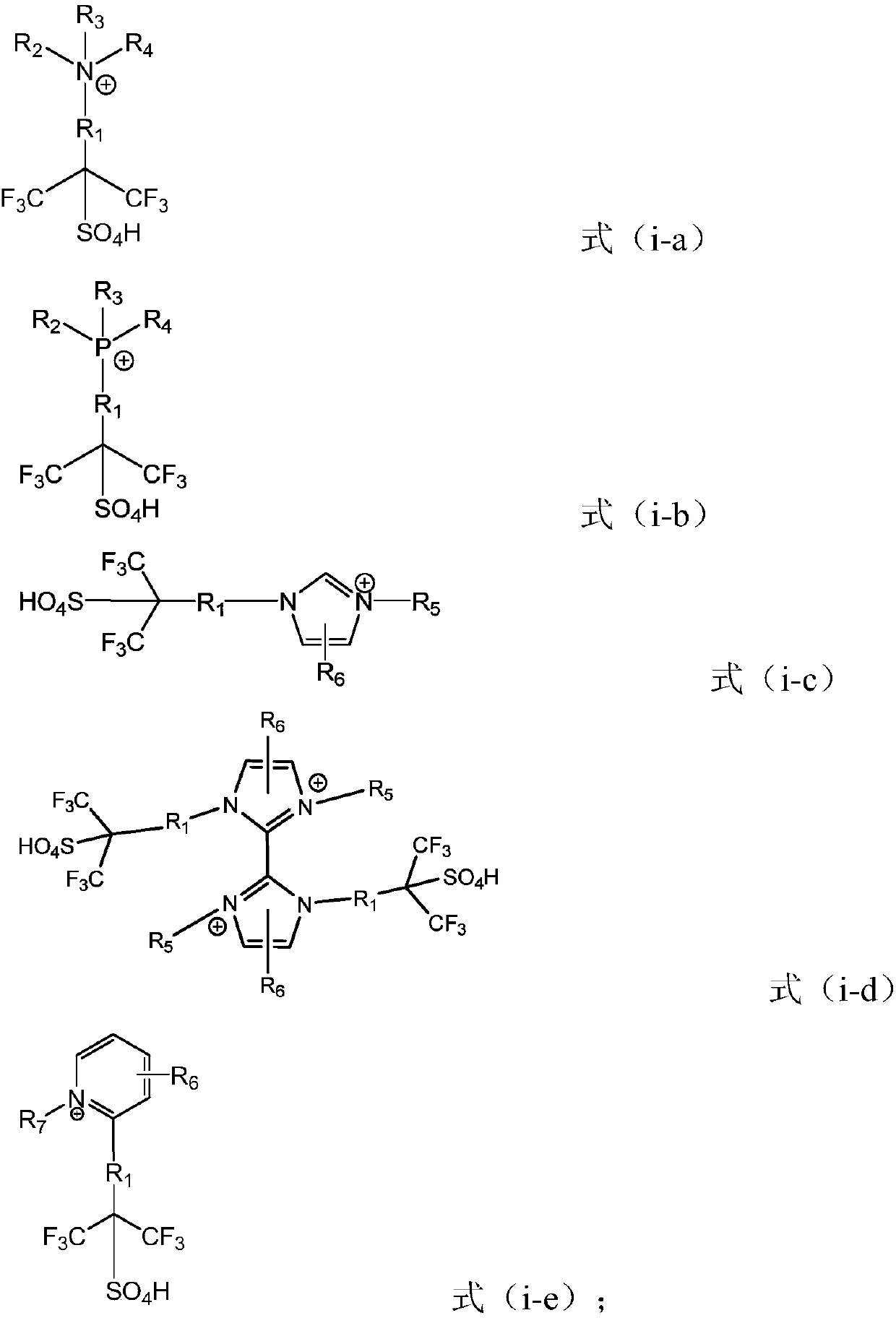
Ionic liquid, preparation method and application
A technology of ionic liquid and cation, which is applied in the preparation of sulfate ester, sulfonate salt, carboxylate ester, etc., and can solve the problems that the strong acid catalytic performance of hexafluoroisopropanesulfonic acid cannot be reflected.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1 Preparation of N,N,N-dimethylbutylhexafluoroisopropanesulfonate ammonium chlorosulfonate (1# ionic liquid)
[0147] The synthetic route is:
[0148]
[0149] The specific steps are:
[0150] (1) Synthesis of N,N-dimethylhexafluoroisopropanol-based amine: Take 0.1 mol of aminohexafluoroisopropanol in a three-necked flask, add 100 mL of isopropanol, stir and heat to 35°C. Then use a constant-pressure dropping funnel to slowly add 0.5 mol of formic acid (the addition is completed in 40 minutes), and the mixture is heated to 50°C; take 0.2 mol of formaldehyde and add it with a constant-pressure dropping funnel under strong stirring (the addition is completed in 4.5 hours); The mixture was heated to 80°C and maintained at reflux for 21h. After cooling, it is alkalized with NaOH solution to pH=13, the solution is separated into layers, the upper layer is extracted with n-hexane, the solvent is evaporated using a rotary evaporator, and the maximum rotary evaporation tempe...
Embodiment 2
[0158] Example 2 Preparation of N, N, N-dimethylbutyl hexafluoroisopropanesulfonate ammonium triflate (2# ionic liquid)
[0159] Step (4) was performed after step (3) of Example 1 to combine the N,N,N-dimethylbutylhexafluoroisopropanesulfonate chlorosulfonate obtained in step (3) with trifluoromethanesulfonic acid Stirring at room temperature for 24h anion ion exchange to obtain N,N,N-dimethylbutyl hexafluoroisopropanesulfonate ammonium triflate.
[0160] Characterization:
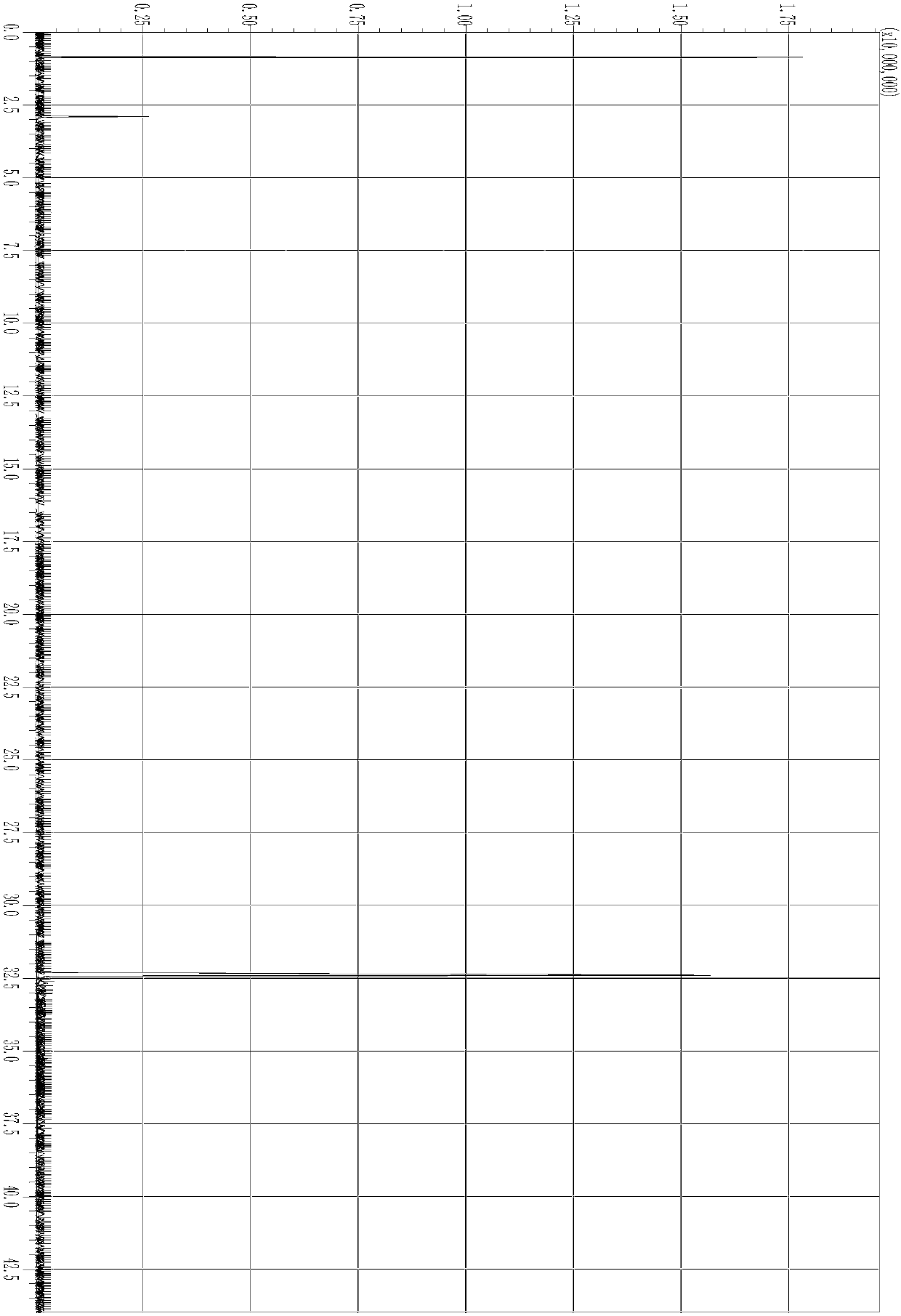
[0161] 1 H NMR(300MHz,[D 6 ]DMSO,25℃):δ=0.89(t,3H,N(CH 2 ) 3 CH 3 ),1.3(m,2H,N(CH 2 ) 2 CH 2 CH 3 ),1.71(m,2H,NCH 2 CH 2 CH 2 CH 3 ),3.3(s,6H,N(CH 3 ) 2 ),3.22(t,2H,NCH 2 (CH 2 ) 2 CH 3 ), 4.2 (s, 1H, OH). ESI / MS: m / z (+) 348.07, m / z (-) 149.
[0162] After testing, the acidity of 2# ionic liquid is H 0 =-9.401.
Embodiment 3 6
[0163] Example 3 Preparation of hexafluoroisopropanesulfonate-disulfonate ammonium triflate (3# ionic liquid)
[0164] The synthetic route is:
[0165]
[0166] The specific steps are:
[0167] At minus 15°C, slowly add 0.1 mol (18.3g) 2-aminohexafluoroisopropanol to 0.4 mol chlorosulfonic acid dropwise and stir while adding dropwise. After the addition is complete, slowly raise the temperature to minus 5°C and stir. After 5h, the temperature was raised to 35℃ and stirred for 5h. The substrate was washed with dichloromethane after the reaction, then vacuum dried, and then slowly added dropwise with one-fold molar amount of trifluoromethanesulfonic acid to the dried bottom at room temperature. Stir while adding dropwise, react for 24h, rotary evaporate the reaction product, and obtain the target product after vacuum drying.
[0168] Characterization:
[0169] 1 H NMR(300MHz,[D 6 ]DMSO,25℃):δ=4.2(s,1H,SO 4 H),7.2(s,1H,NH),8.5(s,2H,2SO 3 H),3.3(s,6H,N(CH 3 ) 2 ),3.22(t,2H,NCH 2 (CH 2 ) 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com