Preparation method of acetoacetamide-N-sulfonic acid triethylamine salt
A technology of triethylamine sulfonate and acetoacetamide, which is applied in the field of preparation of acetoacetamide-N-triethylamine sulfonate, can solve the problems of unsatisfactory large-scale continuous production, slow reaction speed, long reaction time, etc. problems, to achieve the effect of simplifying the product post-processing process, good product appearance, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of acetoacetamide-N-sulfonic acid triethylamine salt of the present application comprises:
[0024] Amination reaction steps: dissolving sulfamic acid in the first dichloromethane to configure the first reaction solution; dissolving triethylamine in the second dichloromethane to configure the second reaction solution, adding the second reaction solution to Amination reaction is carried out in the first reaction solution to form ammonium sulfamate solution.
[0025] At first, be the preparation of sulfamic acid ammonium salt, specifically, sulfamic acid and triethylamine are dissolved in methylene chloride respectively, sulfamic acid and triethylamine exothermic reaction, in the reaction process, the heat that produces will Part of the dichloromethane is vaporized, and the vaporized dichloromethane will leave the reaction system to take away the heat produced. Further, the vaporized dichloromethane can also be recycled.
[0026] The first reaction...
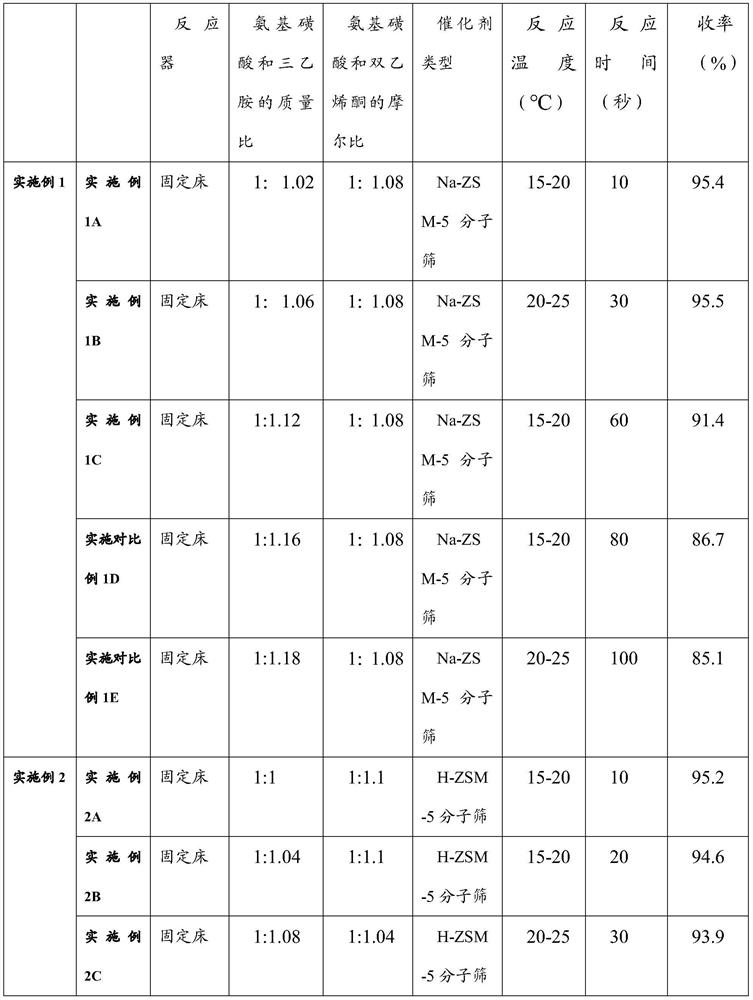
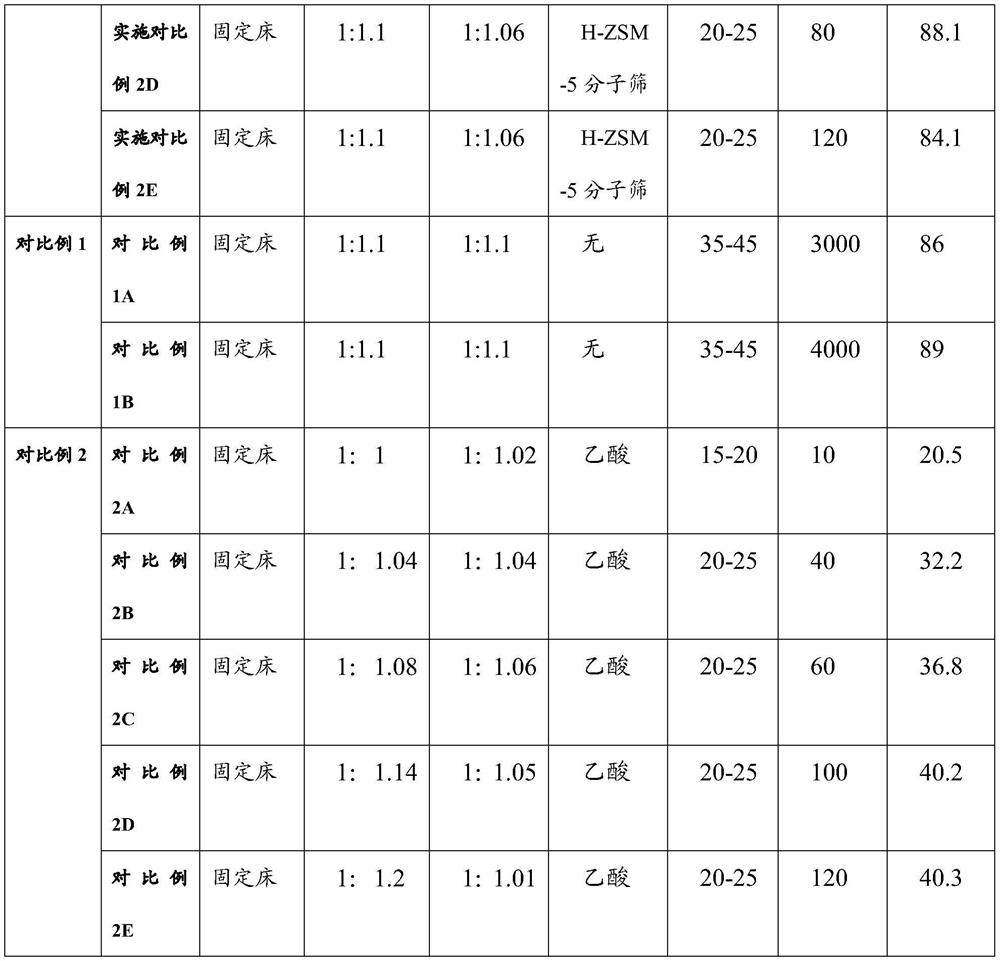
Embodiment 1
[0059] Embodiment 1 (comprising embodiment 1A, embodiment 1B, embodiment 1C, implementing comparative example 1D, implementing comparative example 1E)
[0060] Amination reaction steps: Dissolve 98kg of sulfamic acid and the first dichloromethane at a molar ratio of 1:6, and control the dissolution temperature at about 20-25°C to obtain the dichloromethane solution of sulfamic acid as the first reaction solution. Dissolution can be in a continuous mixing device or in a reactor. Dissolving triethylamine and dichloromethane in a molar ratio of 1:1, controlling the dissolution temperature to be 10-30°C, to obtain a second reaction solution, wherein the mass ratio of sulfamic acid to triethylamine is 1: 1-1.2. Gradually drop the second reaction liquid into the reaction kettle where the first reaction liquid is located for mixing and stirring, and control the system to be weakly alkaline. After mixing evenly, the ammonium sulfamate solution is obtained.
[0061] Embodiment 1A,...
Embodiment 2
[0067] Embodiment 2 (comprising embodiment 2A, embodiment 2B, embodiment 2C, implementing comparative example 2D, implementing comparative example 2E)
[0068] Amination reaction steps: Dissolve 98kg of sulfamic acid and the first dichloromethane at a molar ratio of 1:15, and control the dissolution temperature at about 20-25°C to obtain the dichloromethane solution of sulfamic acid as the first reaction solution. Dissolution can be in a continuous mixing device or in a reactor. Dissolving triethylamine and dichloromethane in a molar ratio of 1:1.2, controlling the dissolution temperature to be 10-30°C, to obtain a second reaction solution, wherein the mass ratio of sulfamic acid to triethylamine is 1: 1-1.2. Gradually drop the second reaction liquid into the reaction kettle where the first reaction liquid is located for mixing and stirring, and control the system to be weakly alkaline. After mixing evenly, the ammonium sulfamate solution is obtained.
[0069] Embodiment ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com