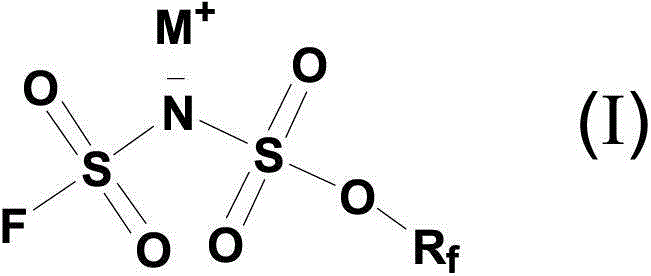
Alkali metal salt of (sulfonyl fluoride)( multi-fluorine alkoxy sulfonyl) imine and ionic liquids
A technology of polyfluoroalkoxysulfonyl and polyfluoroalkyl, which is used in the synthesis of imine alkali metal salts and ionic liquids, and can solve problems such as strong corrosion, inapplicability to large-scale preparation, and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
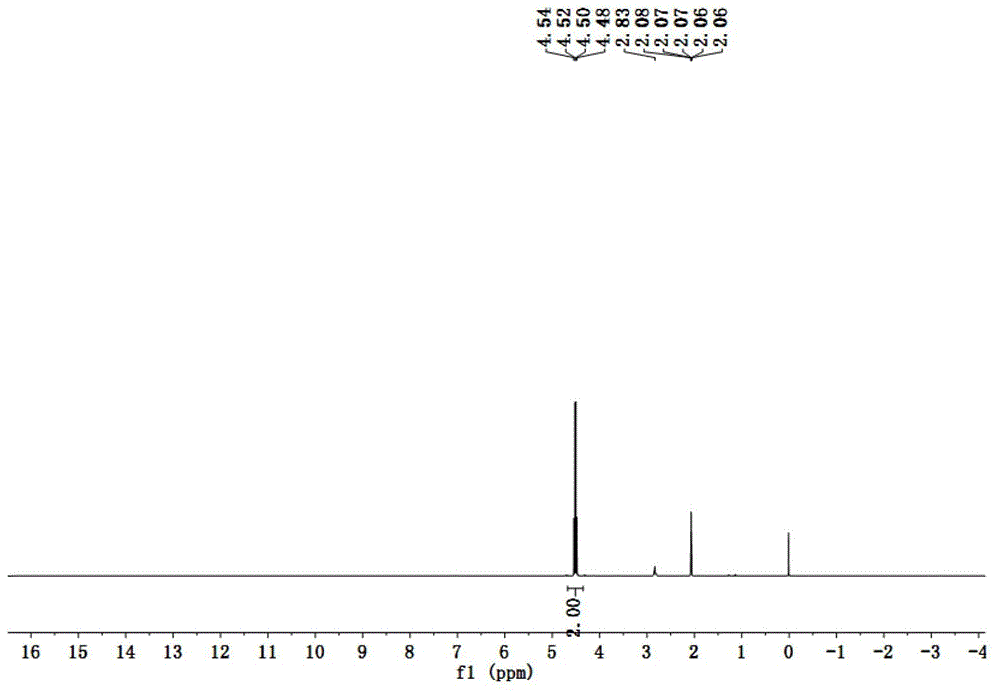
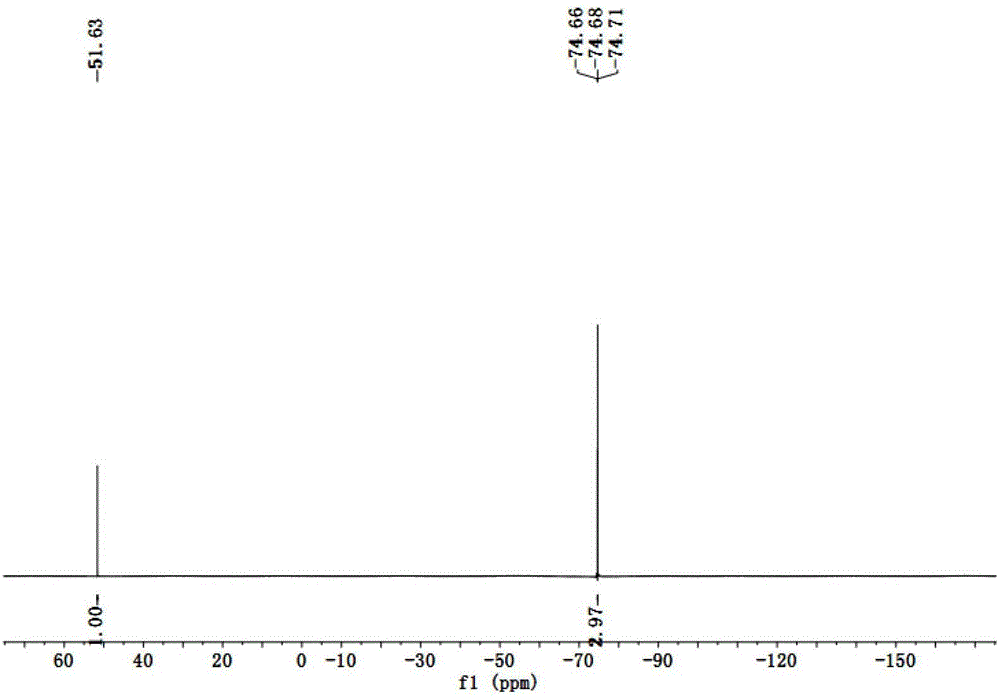
[0034] Embodiment 1: Potassium (fluorosulfonyl) (2,2,2-trifluoroethoxysulfonyl)imide (K[(FSO 2 )(CF 3 CH 2 OSO 2 )N], K[TFE-FSI]) synthesis
[0035] Mix 100g (1.0mol) trifluoroethanol (CF 3 CH 2 OH), 120g (1.2mol) triethylamine mixed system, slowly drop 92.4g (0.8mol) chlorosulfonamide (ClSO 2 NH 2 ) and 50mL of ethyl acetate, reflux for 20h, acidify with concentrated hydrochloric acid, extract with ether, and evaporate organic matter with a low boiling point under reduced pressure to obtain a colorless solid that is 2,2,2-trifluoroethoxysulfonamide ( CF 3 CH 2 OSO 2 NH 2 ) 143g, yield 80%.
[0036] 89.5g (0.5mol) CF 3 CH 2 OSO 2 NH 2 Add 100mL of acetonitrile to dissolve it, slowly add 67.5g (0.5mol) of sulfuryl chloride dropwise in an ice-salt bath, and react in the dark at 25°C for 20h, add 48g (0.3mol) of pyridine-hydrogen fluoride ((C 5 h 5 N)-(HF) 4 ), reacted at 60°C for 20h. Stop the reaction, add base K in ice bath 2 CO 3 Neutralize to pH 7-8, col...
Embodiment 2-11
[0037] Examples 2-11: According to the reaction conditions and operation sequence of Example 1, the preparation conditions and results of other alkali metal salts are shown in Table 1.
[0038]Table 1. Partial synthesis conditions and yields based on (fluorosulfonyl) (2,2,2-trifluoroethoxysulfonyl) imide anion alkali metal salts (SO in the table 2 Cl 2 The dosage is 0.5mol)
[0039]
[0040]
[0041] Note: [TFE-FSI] - =[(FSO 2 )(CF 3 CH 2 OSO 2 )N] - ;
Embodiment 12
[0043] Embodiment 12: Ionic liquid [IM(CH 3 )(CH 2 CH 3 )][TFE-FSI]([TFE-FSI] - =[(FSO 2 )(CF 3 CH 2 OSO 2 )N] - )
[0044] By K[TFE-FSI] and Im(CH 3 )(CH 2 CH 3 ) Br prepared at room temperature. The specific operation is as follows: 6.3g (21mmol) K[TFE-FSI] and 3.80g (20mmol) IM (CH 3 )(CH 2 CH 3 )Br were dissolved in appropriate amount of deionized water respectively, then mixed at room temperature, and after 30 minutes of magnetic stirring reaction, the stratification was allowed to stand, and the lower layer liquid was separated with a separatory funnel. The lower layer liquid was the crude ionic liquid, and the crude product was dissolved in Dichloromethane was washed three times with deionized water, and the dichloromethane solvent was removed under reduced pressure, and dried under reduced pressure at 90° C. for 12 hours to obtain 6.30 g of a colorless transparent liquid with a yield of 85%. 1 H NMR (400MHz; CDCl 3 ;TMS): 1.57(t, J=7.2Hz, 3H), 4.04(s, 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com