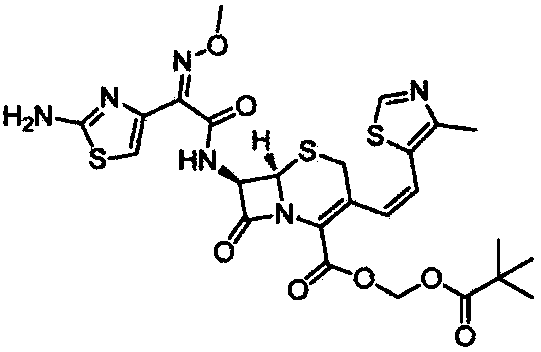
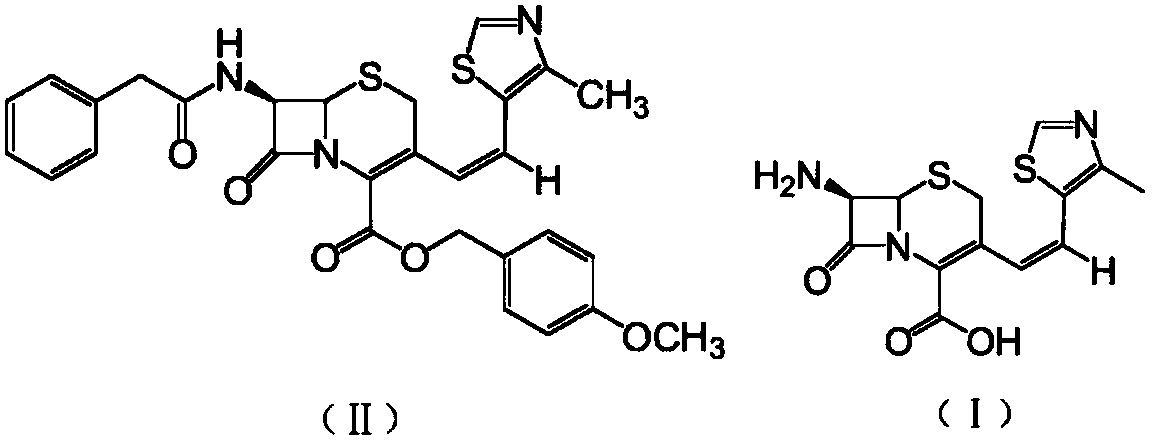
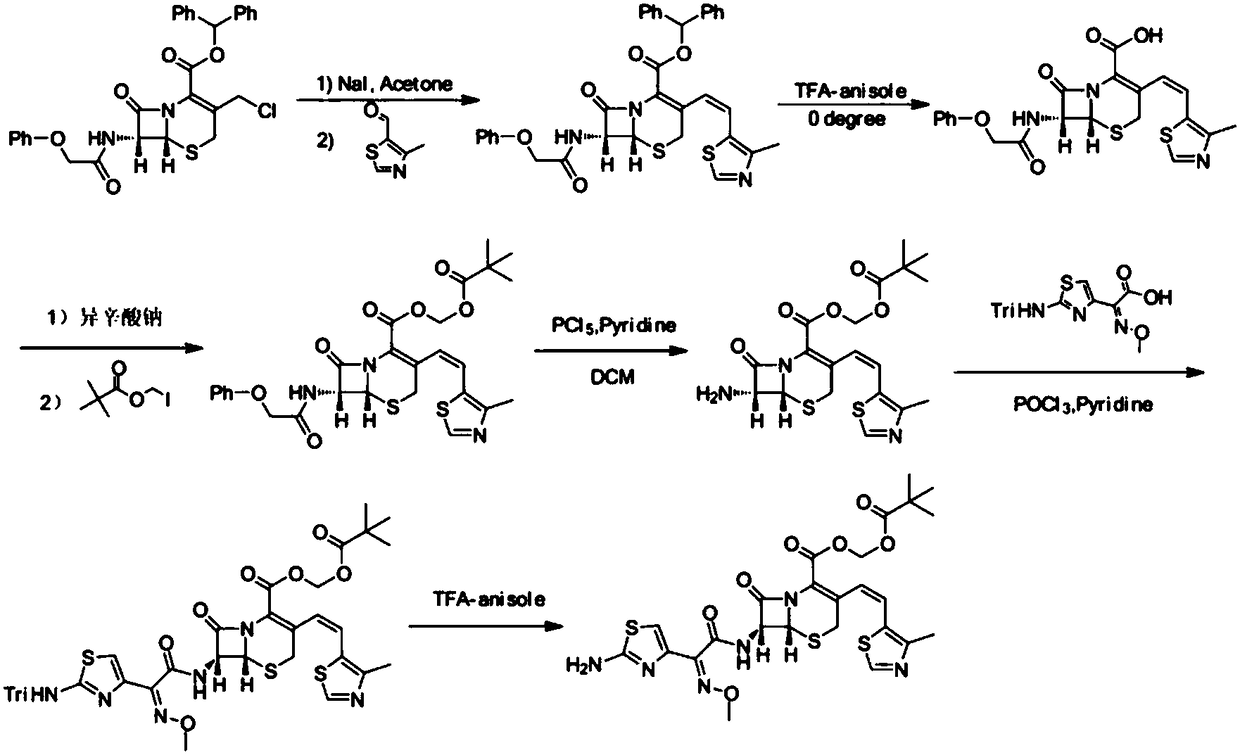
Preparation method of Cefditoren Pivoxil
A technology of cefditoren pivoxil and its compounds, which is applied in the field of preparation of cefditoren pivoxil, can solve problems such as yield reduction, messy reaction, difficult purification process, etc., and achieve increased reactivity, mild reaction conditions, and product purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Step 1) Under nitrogen protection, 10.89g 3-acetoxymethyl-5-thio-7-amino-8-oxo-1-azabicyclooct-2-ene-2carboxylic acid, 100mL acetonitrile, 46mmol N , put O-bistrimethylsilylacetamide (BSA) in the reaction bottle, stir the reaction at room temperature for 4h, add dropwise N,N-diethylaniline 4~6mL, trimethyl iodosilane (TMSI) 44mmol, in 10~ React at 15°C for 1 hour, add 11.02 g of triphenylphosphine, continue the reaction for 1 hour, add 7.34 g of sodium hexamethyldisilazide, stir at room temperature for 45 minutes, separate the organic layer and wash with water, then wash with 20% w / w NaCl aqueous solution, Anhydrous MgSO 4 After drying, 22.01 g of compound 4 was obtained, with a yield of 89%.
[0038] Step 2)
[0039] [C 4 MIm]PF 6 Preparation of ionic liquid: 6.896g KPF will be dissolved 6 50mL of acetone solution was placed in a 250mL three-necked flask, and then 10mL containing 5.47g [C 4 MIm]Br in acetone solution, a white precipitate appeared immediately, aft...
Embodiment 2
[0044] Step 1) Under nitrogen protection, 10.89g of 3-acetoxymethyl-5-thio-7-amino-8-oxo-1-azabicyclooct-2-ene-2carboxylic acid, 100mL of acetonitrile, 40mmol N , put O-bistrimethylsilylacetamide (BSA) in the reaction bottle, stir the reaction at room temperature for 4h, add dropwise N,N-diethylaniline 4~6mL, trimethyl iodosilane (TMSI) 40mmol, in 10~ React at 15°C for 1 hour, add 10.49 g of triphenylphosphine, continue the reaction for 1 hour, add 7.34 g of sodium hexamethyldisilazide, stir at room temperature for 45 minutes, separate the organic layer and wash with water, then wash with 20% w / w NaCl aqueous solution, Anhydrous MgSO 4 After drying, 19.79 g of compound 4 was obtained, with a yield of 80%.
[0045] Step 2)
[0046] [C 4 MIm]PF 6 Preparation of ionic liquid: 6.896g KPF will be dissolved 6 50mL of acetone solution was placed in a 250mL three-necked flask, and then 10mL containing 5.47g [C 4 MIm]Br in acetone solution, a white precipitate appeared immediatel...
Embodiment 3
[0051] Step 1) Under nitrogen protection, 10.89g of 3-acetoxymethyl-5-thio-7-amino-8-oxo-1-azabicyclooct-2-ene-2carboxylic acid, 100mL of acetonitrile, 52mmol N , put O-bistrimethylsilylacetamide (BSA) in the reaction bottle, stir the reaction at room temperature for 4h, add dropwise N,N-diethylaniline 4~6mL, trimethyl iodosilane (TMSI) 40mmol, in 10~ React at 15°C for 1 hour, add 11.54 g of triphenylphosphine, continue the reaction for 1 hour, add 7.34 g of sodium hexamethyldisilazide, stir at room temperature for 45 minutes, separate the organic layer and wash with water, then wash with 20% w / w NaCl aqueous solution, Anhydrous MgSO 4 After drying, 20.78 g of compound 4 was obtained, with a yield of 84%.
[0052] Step 2)
[0053] [C 4 MIm]PF 6 Preparation of ionic liquid: 6.896g KPF will be dissolved 6 50mL of acetone solution was placed in a 250mL three-necked flask, and then 10mL containing 5.47g [C 4 MIm]Br in acetone solution, a white precipitate appeared immediatel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com