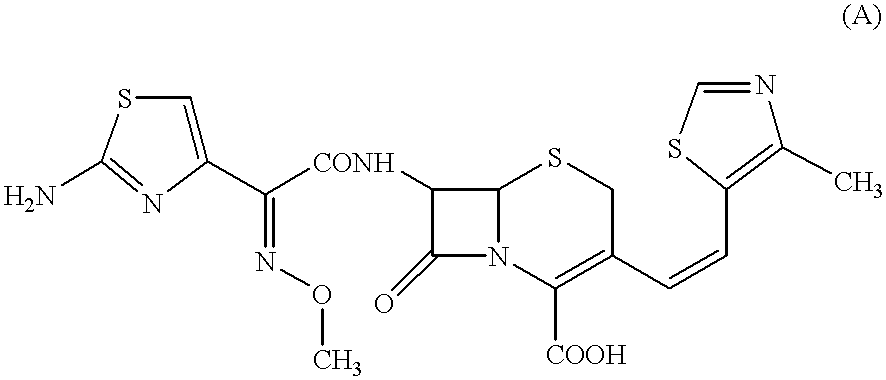
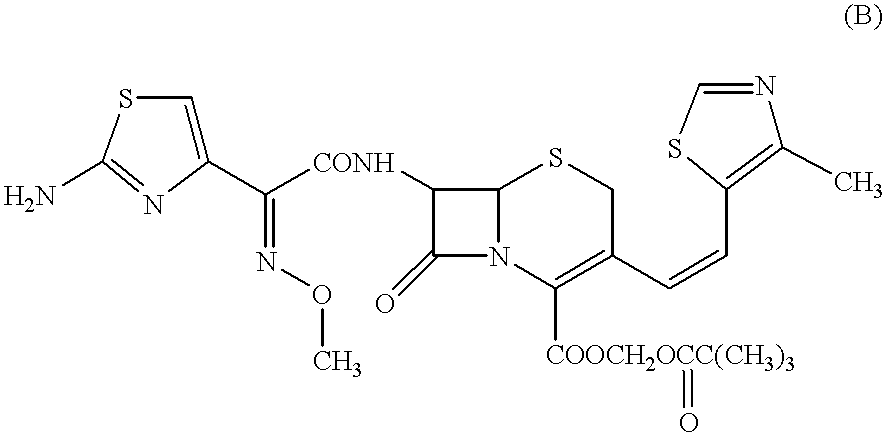
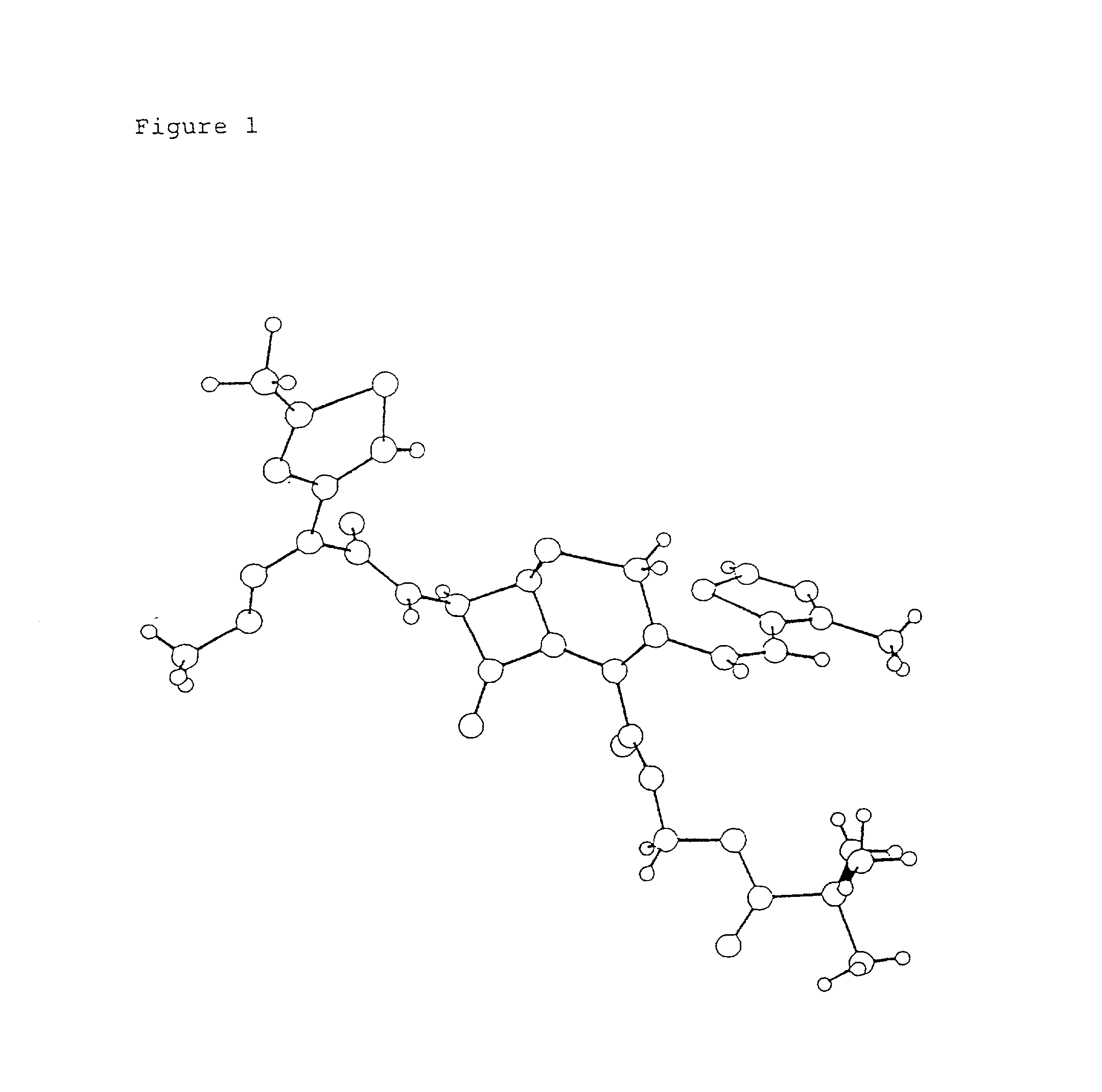
Crystalline substance of cefditoren pivoxyl and the production of the same
a technology of cefditoren pivoxyl and crystalline substance, which is applied in the field of cefditoren pivoxyl crystalline substance and the production of the same, can solve the problems of not being stable enough and not yet a completely satisfactory drug, and achieve the effect of facilitating the crystalline substance of cefditoren pivoxyl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0076] An amorphous substance (10 g) of Cefditoren pivoxyl, namely 7-[(Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido]-3-[(Z)-2-(4-methy-lthiazol-5-yl)-ethenyl]-3-cephem-4-carboxylic acid pivaloyloxymethyl ester, was dissolved in ethyl acetate (400 ml) at room temperature (at 10.degree. C.). The resulting solution containing the dissolved Cefditoren pivoxyl at a concentration of 25 mg / ml of ethyl acetate was then mixed with anhydrous ethanol (60 m) at a temperature of 5.degree. C. or below, to prepare a solution containing 217 mg / ml of Cefditoren pivoxyl in the mixture of ethyl acetate and ethanol (totally 460 ml). This solution was concentrated to a volume of 80 ml by evaporation of ethyl acetate and ethanol under a reduced pressure of 20 Torr (in guage), with keeping the temperature of said solution below 10.degree. C.
[0077] The concentrated solution so obtained had a concentration of 125 mg / ml of Cefditoren pivoxyl dissolved in a mixture of a larger proportion of ethanol and ...
examples 2-3
[0083] The procedures of Example 1 above were repeated by using methanol in place of ethanol.
[0084] The procedures of Example 1 were again repeated by using iso-propanol in place of ethanol.
[0085] In these two runs of the experiments, there were obtained pale yellow crystals of Cefditoren pivoxyl in yields of 7.6 g and 7.8 g, respectively. The two crop products of the crystalline Cefditoren pivoxyl were found to be of the orthorhombic form and have a purity of 98% and a purity of 97%, respectively.
example 4
[0086] An amorphous substance (10 g) of Cefditoren pivoxyl was dissolved in ethyl acetate (400 ml) at room temperature (at 10.degree. C.). The resulting solution containing the dissolved Cefditoren pivoxyl at a concentration of 25 mg / ml in ethyl acetate was then added with 0.02 g of a previously prepared crystalline substance of Cefditoren pivoxyl obtained in Example 1, as the seed crystal.
[0087] The solution of Cefditoren pivoxyl in ethyl acetate containing the seed crystal added was incubated at 10.degree. C. for 40 hours under mechanical agitation. After this incubation, this solution was concentrated to a volume of 40 ml under a reduced pressure of 20 Torr, with keeping the temperature of the solution below 10.degree. C. The concentrated solution so obtained contained 250 mg / ml of the dissolved Cefditoren pivoxyl in ethyl acetate along with the seed crystal.
[0088] This concentrated solution in ethyl acetate was then mixed with ethanol (60 ml) to prepare a solution containing 100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com