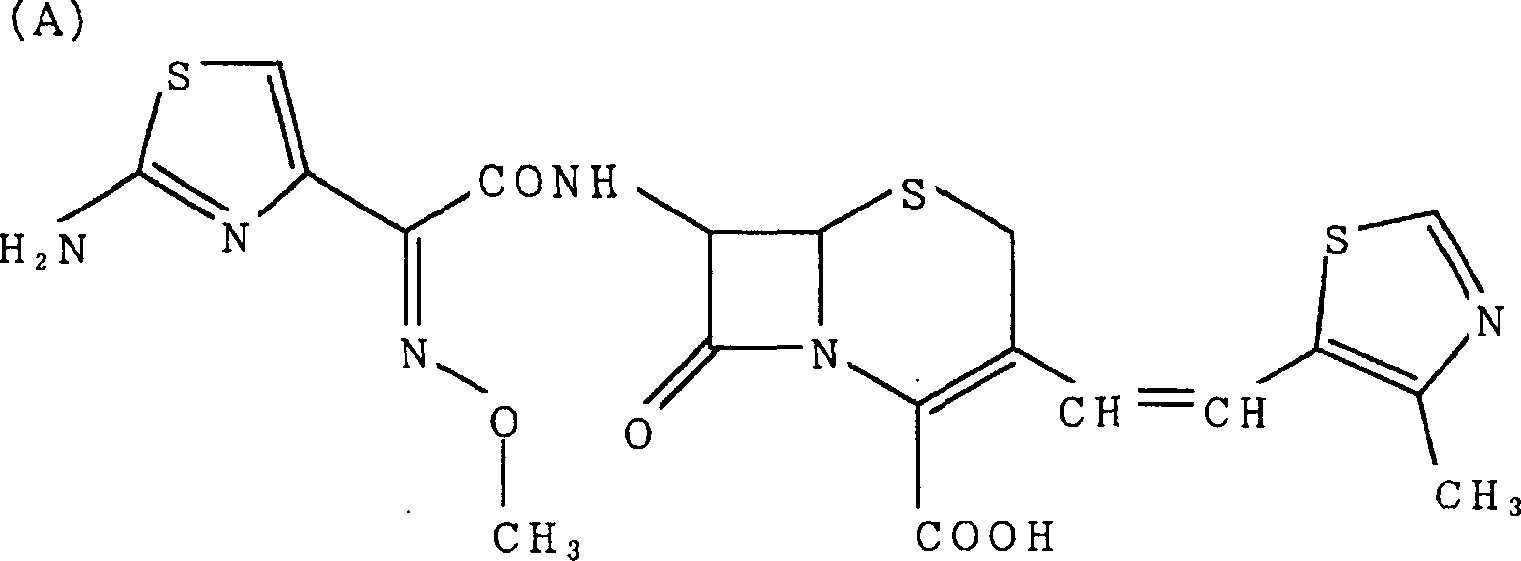
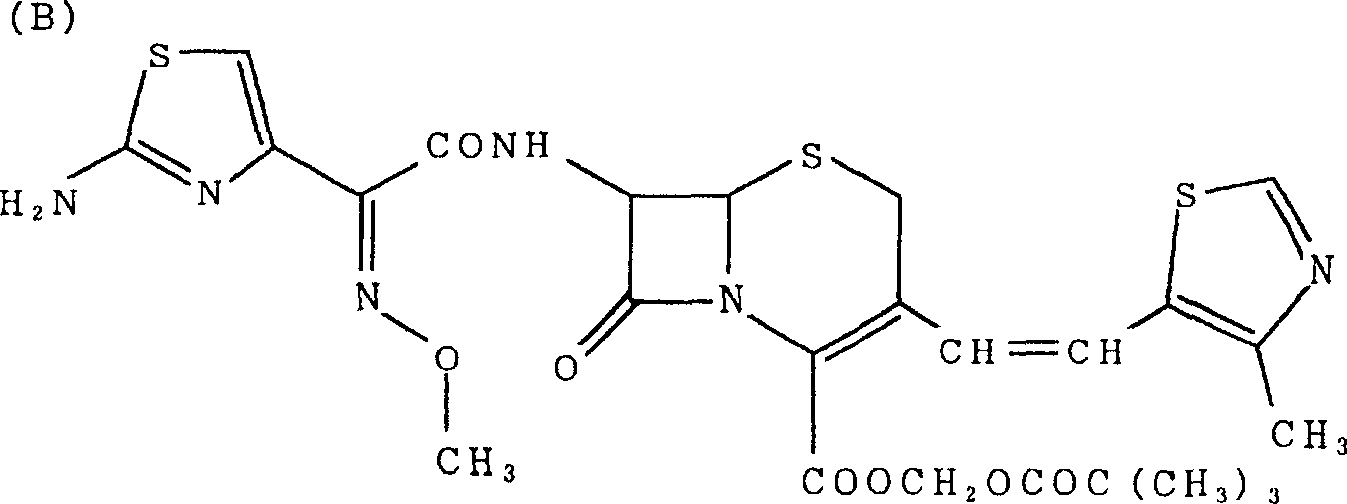
Noncrystalline antibacterial composition containing cefditoren pivoxil
A kind of technology of cefditoren and composition, applied in the field of amorphous antibacterial composition, can solve problems such as inability to be administered to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Examples 1, 6, 7 and 8: Solid Dispersions Containing Surfactants
[0044] The solid dispersion composition was obtained by dissolving crystalline cefditoren pivoxil and surfactant in a mixture of dichloromethane:methanol (1:1) at the preparation ratio shown in Table 1, and then removing the solvent by distillation . The amorphous form of cefditoren pivoxil in these compositions was confirmed by powder X-ray diffraction analysis (data omitted). Crystalline cefditoren pivoxil was prepared according to Japanese Patent No.3403206.
[0045] Surfactant
Preparation ratio
(drug: surfactant)
Example 1
fatty acid sucrose ester
100mg Potency: 5.0mg
Example 6
fatty acid sucrose ester
100mg Potency: 0.01mg
Example 7
fatty acid sucrose ester
100mg Potency: 0.1mg
Example 8
fatty acid sucrose ester
100mg Potency: 200mg
[0046] Fatty acid sucrose ester: DK Ester SS, HLB value = 20, Daiichi Kogy...
preparation Embodiment 1
[0062] A powdery formulation was prepared by mixing 130 g of the composition obtained in Example 1 with 260 g of cornstarch.
preparation Embodiment 2
[0064] 1000 capsules were prepared by mixing 130 g of the composition obtained in Example 4, 260 g of spray-dried lactose, 130 g of croscarmellose sodium and 3 g of magnesium stearate, and then filling the mixture into capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com