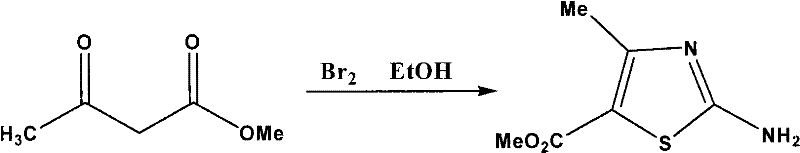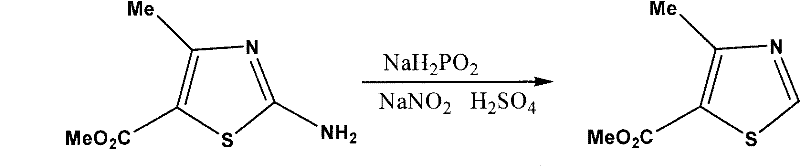Synthetic technology of cefditoren pivoxil intermediate
A cefditoren pivoxil and synthetic process technology, applied in the direction of organic chemistry, etc., can solve the problems of long synthetic steps, environmental pollution, difficult to handle by-product chromium salts, etc., and achieve mild reaction conditions, easy post-treatment, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Processing step of the present invention is:
[0021] 1. Preparation of methyl 2-amino-4-methyl-5-thiazolecarboxylate
[0022] The reaction equation is:
[0023]
[0024] 2. Preparation of methyl 4-methyl-5-thiazolecarboxylate
[0025] The reaction equation is:
[0026]
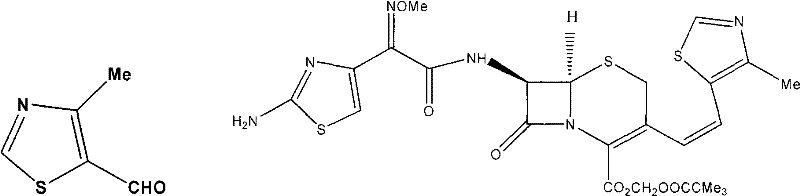
[0027] 3. Preparation of 4-methyl-5-formylthiazole
[0028] The reaction equation is:
[0029]
[0030] The product of the present invention has been confirmed by nuclear magnetic spectrum and mass spectrogram.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com