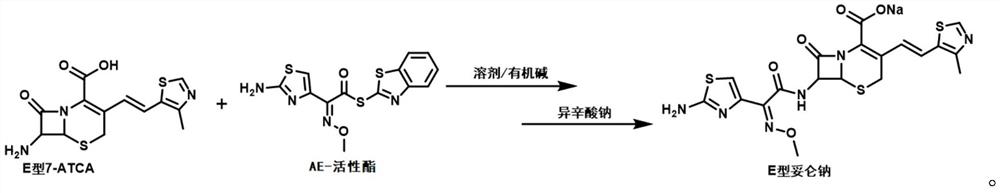
Preparation method of E-type cefditoren sodium
The technology of cefditoren sodium and sodium isooctanoate is applied in the field of preparation of E-type cefditoren sodium, which can solve the problem of poor activity of E-type 7-ATCA, low purity and yield of E-type cefditoren sodium, and inability to control impurities. product use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
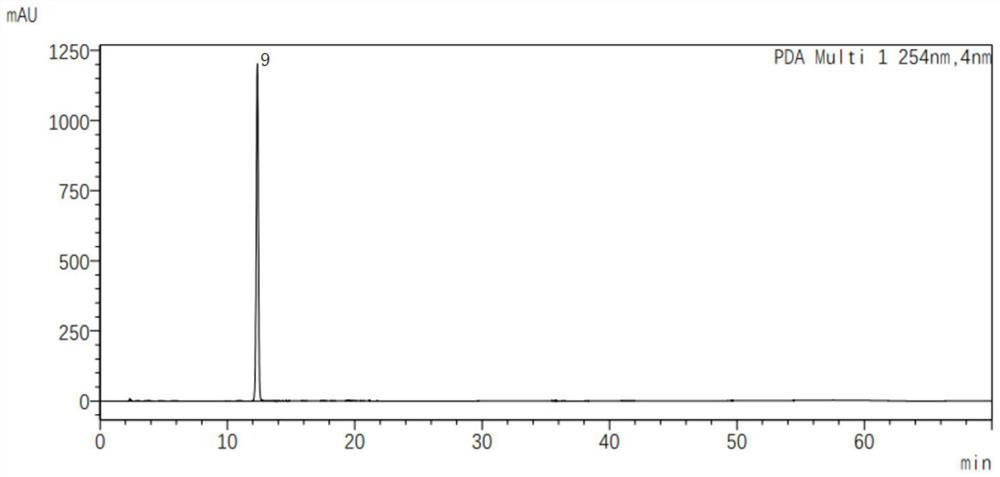
[0025] Add 6.6mL of water and 30mL of acetone into the reaction vessel, cool down to 0°C, add 3g (9.28mmol) of E-type 7-ATCA and 3.25g (9.28mmol) of AE active ester (MAEM), and dropwise add triethylamine to make the reaction liquid The pH value of the solution reached 8.0, and then the temperature was raised to 15°C for reaction. During the reaction, the pH of the reaction solution was maintained at 8.0-8.5 by adding triethylamine. After the reaction was complete (3h), 1.54g of sodium octanoate (9.28mmol) was added. 45mL of acetone solution, the crystal was grown for 1 hour, and a light yellow solid was obtained by suction filtration. The light yellow solid that obtains is transferred to 25 ℃ of vacuum dryings in oven for 5 hours, obtains 2.78g dry product, detects through HPLC, and the purity of E type cefditoren sodium in the dry product is 98.6%, and detection result is as follows: figure 1 Shown, the mass yield of the calculated E-type cefditoren sodium is 91.4%.
[0026]...
Embodiment 2
[0031] Add 4.5mL of water and 15mL of acetone to the reaction vessel, cool down to 5°C, add 3g (9.28mmol) of E-type 7-ATCA and 4.87g (13.92mmol) of AE active ester (MAEM), and dropwise add diethylamine to make the reaction liquid The pH value of the solution reaches 8.5, and then the temperature is raised to 20°C for reaction. During the reaction, the pH of the reaction solution is maintained at 8.0-8.5 by adding diethylamine. After the reaction is complete (2.5h), add 2.31g of sodium isooctanoate (13.92mmol ) of 45 mL of acetone solution, crystal growth for 1.5 hours, and suction filtration to obtain a pale yellow solid. The light yellow solid that obtains is transferred to 35 ℃ of vacuum dryings in the oven for 3 hours, obtains 2.75g dry product, detects by HPLC, the purity of E-type cefditoren sodium in the dry product is 98.3%, the calculated E-type cefditoren The mass yield of len sodium is 90.1%.
Embodiment 3
[0033] Add 6mL of water and 20mL of acetone into the reaction vessel, cool down to 2°C, add 3g (9.28mmol) of E-type 7-ATCA (9.28mmol) and 3.90g (11.14mmol) of AE active ester (MAEM), and drop diisopropylethylamine to make the reaction The pH value of the solution reaches 8.2, and then the temperature is raised to 20°C for reaction. During the reaction, the pH of the reaction solution is maintained at 8.0-8.5 by adding diisopropylethylamine. After the reaction is complete (3.5hh), 1.85g of isooctanoic acid Sodium (11.14 mmol) in 45 mL of acetone solution was grown for 1.2 hours, and a light yellow solid was obtained by suction filtration. The light yellow solid that obtains is transferred to 30 ℃ of vacuum dryings in the oven for 4 hours, obtains 2.88g dry product, detects by HPLC, the purity of E-type cefditoren sodium in the dry product is 98.1%, the calculated E-type cefditoren The mass yield of len sodium is 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com