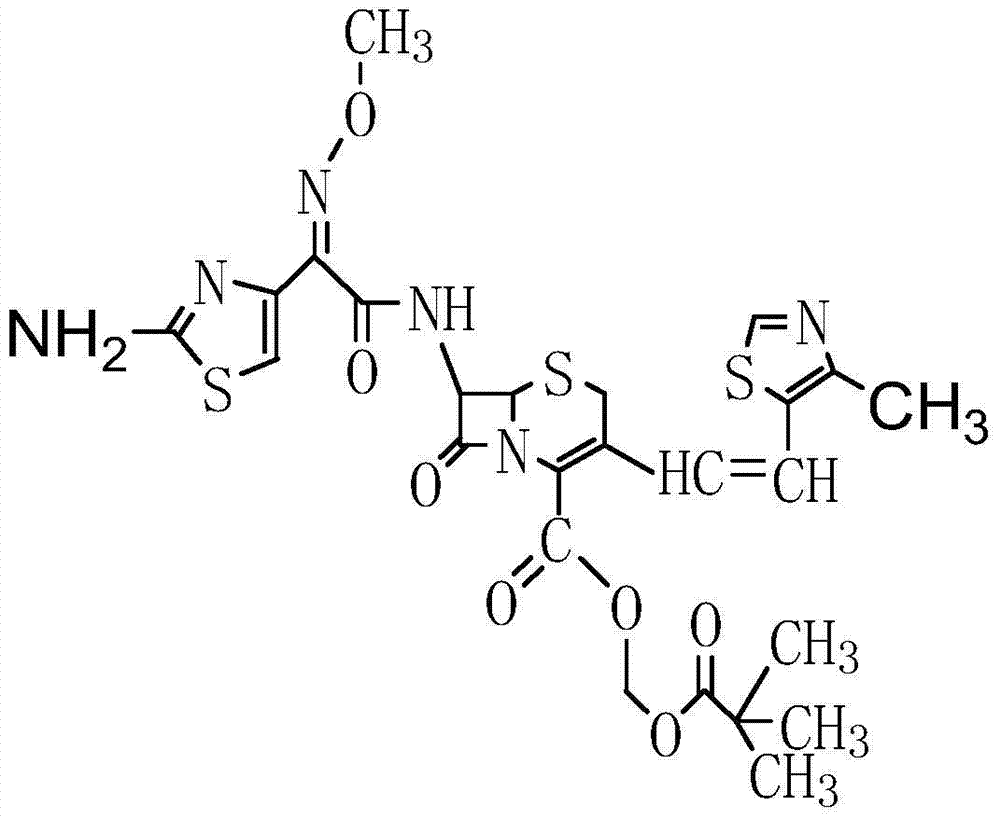
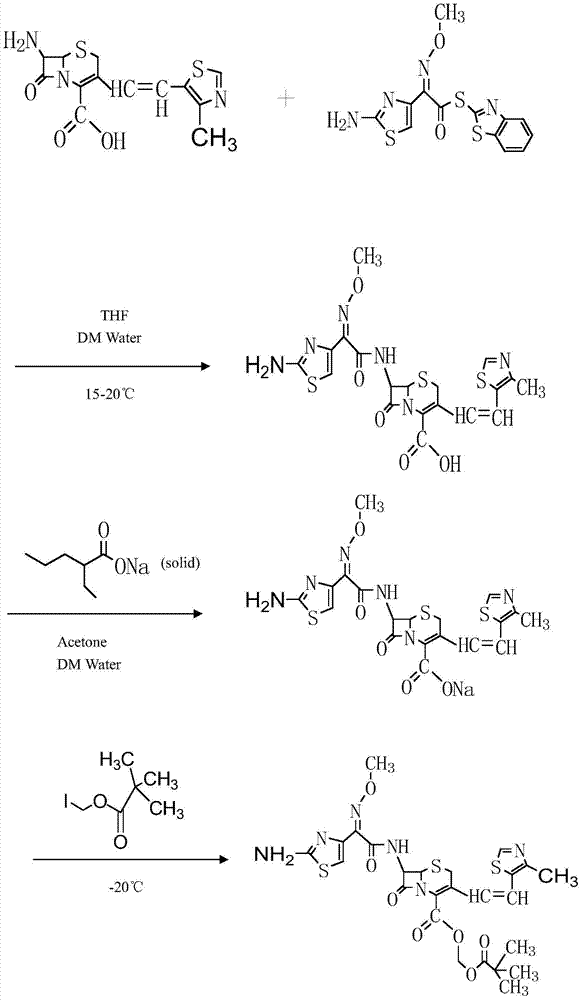
Preparation method of cefditoren pivoxil
A technology of cefditoren pivoxil and cefditoren sodium, which is applied in the field of preparation of cefditoren pivoxil, can solve the problems of high reaction temperature, large amount of iodomethyl pivalate, poor purity stability, etc., and achieve process operation Simple, cheap and easy to obtain, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of embodiment 1 cefditoren pivoxil (small test process)
[0036] Step 1, cefditoren sodium reaction
[0037] At room temperature and protected from light, add 200g of 7ATCA and 260g of AE-active ester into 2000ml of dichloromethane, add 110ml of methanol and 12.6ml of sulfurous acid, stir and cool down to 0°C to 5°C, slowly add 126ml of triethylamine dropwise, and finish adding Afterwards, heat-preserve at 0°C to 5°C for 4 hours, and sample HPLC at 3 hours (the remaining 7ATCA<0.5% means the reaction is complete), the result shows that the reaction is complete, add 400ml of pure water to the reaction solution, stir for 15min, separate the liquid, collect the water phase, and Heat the water phase to 20°C-25°C, add sodium isooctanoate / acetone solution prepared by 200g sodium isooctanoate and 2300ml acetone dropwise into the water phase, keep stirring at 20°C-25°C for 2 hours, filter, the filter cake is below 40°C After drying under reduced pressure...
Embodiment 2
[0042] The preparation method of embodiment 2 cefditoren pivoxil (industrialization process)
[0043] Step 1, cefditoren sodium reaction
[0044] Under the condition of avoiding light at room temperature, add 240L dichloromethane into the 500L glass-lined reaction kettle, start stirring, then add 24.0kg7ATCA, 31.2kg AE-active ester, 13.2L methanol, 1512ml sulfurous acid into the kettle, and cool it with -15℃ Cool the brine to 0°C-5°C, add 15.1L triethylamine to the kettle, control the temperature not to exceed 5°C during the period, keep stirring at 0°C-5°C for 4 hours after the addition is complete. After 4 hours, add 96L of pure water into the kettle, stir for 20 minutes, then let it stand for 20 minutes, separate the liquid, collect the water phase, add the water phase to the glass-lined reaction kettle, stir, and heat up to 20°C to 25°C with process hot water. Add sodium isooctanoate / acetone solution prepared by 24.0kg sodium isooctanoate and 100L acetone from the high-le...
Embodiment 3
[0049] Embodiment 3 step one, cefditoren sodium reaction, reaction temperature is-5 ℃~0 ℃
[0050] Under the condition of avoiding light at room temperature, add 100g of 7ATCA and 130g of AE-active ester into 1000ml of dichloromethane, add 55ml of methanol and 6.3ml of sulfurous acid, stir and cool down to -5℃~0℃, slowly add 63ml of triethylamine dropwise, add After completion, the reaction was incubated at -5°C to 0°C for 4 hours. At 3 hours, HPLC was sampled (remaining 7ATCA<0.5% was the reaction was complete), and the result showed that the reaction was not complete. At 4 hours, the reaction was sampled by HPLC, and the result showed that the reaction was complete. Add 400ml pure water, stirred for 15 minutes, separated, collected the water phase, raised the temperature of the water phase to 20°C to 25°C, and added dropwise the sodium isooctanoate / acetone solution prepared by 100g sodium isooctanoate and 1150ml acetone to the water phase 25 Stir at ℃~30℃ for 2 hours, filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com