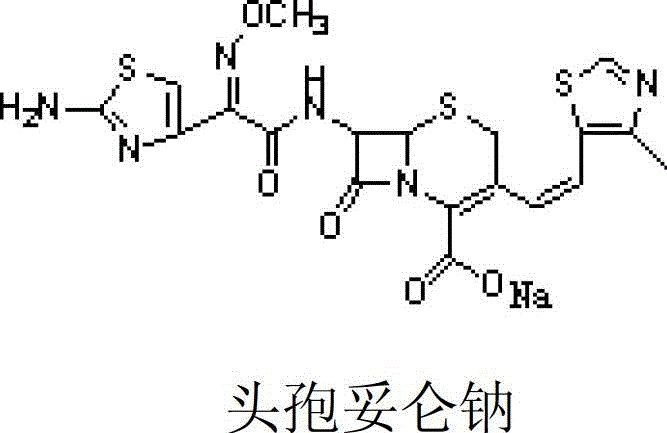
Freeze-dried preparation of cefditoren sodium for injection and preparation method thereof
A technology of cefditoren sodium and freeze-dried preparation, which is applied in the field of cefditoren sodium freeze-dried preparation for injection and its preparation field, can solve the problems of long dissolution time and poor stability of active ingredients, shorten dissolution time and improve stability , good solubilizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
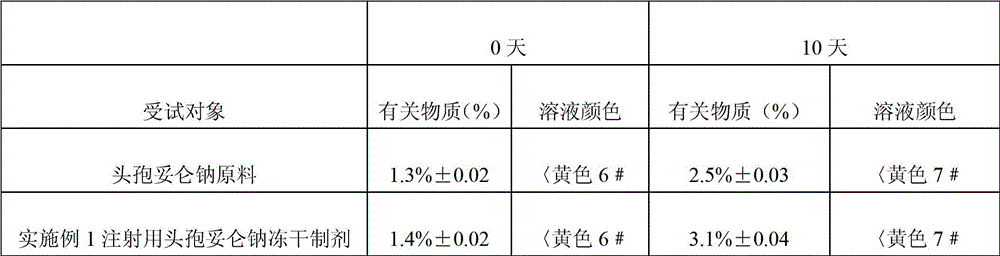
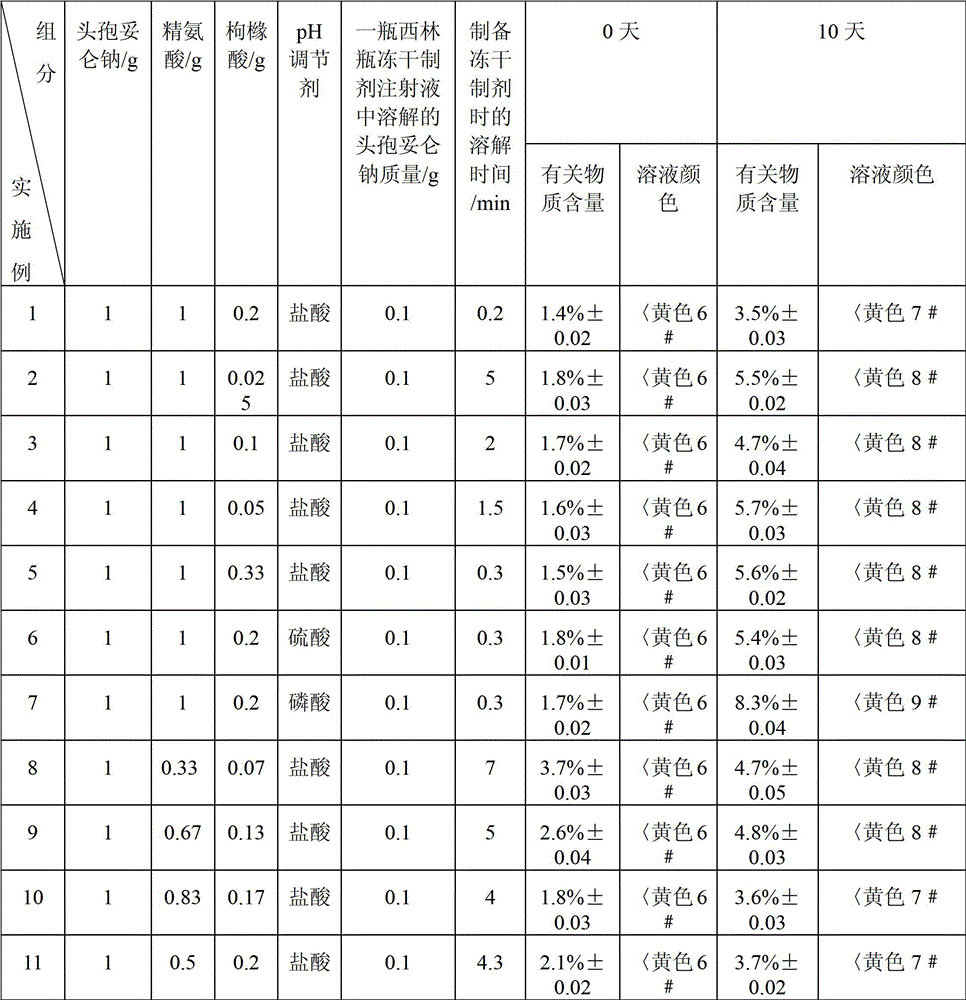
[0023] Example 1 The freeze-dried preparation of cefditoren sodium for injection consists of 1 g of cefditoren sodium, 1 g of arginine, 0.2 g of citric acid and an appropriate amount of hydrochloric acid as a pH regulator.
[0024] The preparation method of this example cefditoren sodium freeze-dried preparation for injection: first dissolve arginine and citric acid with 50mL water for injection, adjust the pH value of the solution to neutrality with hydrochloric acid, then add the sterile cefditoren sodium raw material, stir After dissolving, add activated carbon; Pack the sterile, pyrogen-free medicinal solution into 10 bottles of vials, and freeze-dry it according to the freeze-drying process. Each bottle of vials contains 0.1 g of cefditoren sodium; the freeze-dried The process is: pre-freeze the liquid medicine to -30°C, evacuate to below 10Pa, and then raise the temperature in stages. The operating conditions of the step-by-step temperature increase are: the first stage: ...
Embodiment 2
[0031] The preparation method of the freeze-dried preparation in Example 2 is the same as the preparation method of the freeze-dried preparation in Example 1, the difference is that the operating conditions of the freeze-drying process in Example 2 are: the first stage: -30 ° C ~ -25 ° C , the heating time is 3h; the second stage: -25℃~-15℃, the heating time is 5h; the third stage: -15℃~25℃, the heating time is 5h, the temperature is raised to 25℃ and then kept for 4h. When preparing the freeze-dried formulation described in Example 2, dissolve the cefditoren sodium pharmaceutical composition with 50 mL of water for injection, and see Table 2 for the dissolution time.
Embodiment 3
[0032] The preparation method of the freeze-dried preparation in Example 3 is the same as the preparation method of the freeze-dried preparation in Example 1, the difference is that the operating conditions of the freeze-drying process in Example 3 are: the first stage: -30 ° C ~ -25 ° C , the heating time is 5h; the second stage: -25°C to -15°C, the heating time is 3h; the third stage: -15°C to 25°C, the heating time is 7h, the temperature is raised to 25°C and then kept for 6h. When preparing the freeze-dried formulation described in Example 3, dissolve the cefditoren sodium pharmaceutical composition with 50 mL of water for injection, and see Table 2 for the dissolution time.
[0033] The preparation method of the freeze-dried preparation of Examples 4-11 is the same as the preparation method of the freeze-dried preparation of Example 1. When preparing the freeze-dried formulations described in Examples 4-11, the cefditoren sodium pharmaceutical composition was dissolved wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com