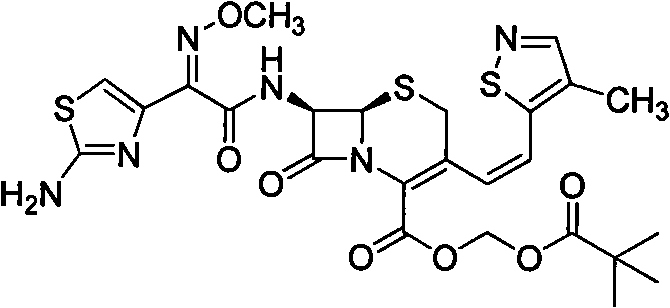
Method for preparing cefditoren pivoxil
A cefditoren axetil and extraction technology, applied in the direction of organic chemistry, antibacterial drugs, etc., can solve the problems of perishable yield, troublesome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Under the condition of avoiding light, 5 g of crude cefditoren axetil with a purity of 96.6% (as determined by HPLC) was dissolved in 100 mL of ethyl acetate at a temperature of 0° C. After washing three times with 40 mL of 1% dilute hydrochloric acid, the aqueous phases were combined, adjusted to pH = 2 with 0.1% wt sodium bicarbonate, and extracted three times with 50 mL of ethyl acetate. The organic phases were combined, washed twice with 100 mL of deionized water, dried with anhydrous sodium sulfate, and treated with activated carbon, the solvent was recovered under reduced pressure, and dried in a vacuum in the dark to obtain cefditoren axetil in a light yellow solid form, with a quality of 4.6 g, purity 98.8%, determined by HPLC.
Embodiment 2
[0023] Under the condition of avoiding light, 5 g of crude cefditoren axetil with a purity of 93.7% (as determined by HPLC) was dissolved in 30 mL of DMF at a temperature of 5° C. After it dissolves, transfer it into a pre-cooled mixed system of 100 mL ethyl acetate and 100 mL deionized water, keep stirring at 0°C for 10 min, and separate the liquids. The organic phases were combined and washed three times with 40 mL of 1% dilute hydrochloric acid, and the aqueous phases were combined, adjusted to pH = 2 with 5% wt sodium bicarbonate, and extracted three times with 50 mL of ethyl acetate. The organic phase was washed with water at a temperature of 5°C, dilute alkali, dilute acid and water, dried with anhydrous sodium sulfate, treated with activated carbon, recovered the solvent under reduced pressure, and dried under vacuum to obtain cefditoren in the form of light yellow solid The ester has a mass of 3.9 g and a purity of 98.1%, determined by HPLC.
Embodiment 3
[0025] Under the condition of avoiding light, 5 g of crude cefditoren axetil with a purity of 93.7% (as determined by HPLC) was dissolved in 30 mL of DMF at a temperature of 5° C. After it dissolves, transfer it into a pre-cooled mixed system of 100 mL ethyl acetate and 100 mL deionized water, keep stirring at 0°C for 10 min, and separate the liquids. The organic phases were combined and washed three times with 40 mL of 1% dilute hydrochloric acid, then the aqueous phases were combined, adjusted to pH=2 with 1% wt of sodium carbonate, and extracted three times with 50 mL of ethyl acetate. The organic phase was washed with water at a temperature of 5°C, dilute alkali, dilute acid and water, dried with anhydrous sodium sulfate, treated with activated carbon, recovered the solvent under reduced pressure, and dried under vacuum to obtain cefditoren in the form of light yellow solid The ester has a mass of 3.8 g and a purity of 98.2%, determined by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com