Preparation methods of Cefditoren acid delta 3 isomer and cefditoren pivoxil delta 3 isomer
A technology of cefditoren pivoxil and cefditoren, which is applied in the field of impurity analysis in drug synthesis, can solve the problems of cumbersome steps, difficult to completely separate impurities, and affect accuracy, and achieve simple steps, guarantee of clinical safe use, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
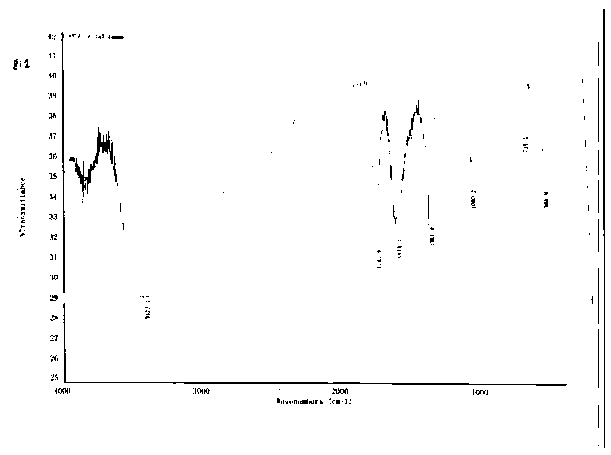
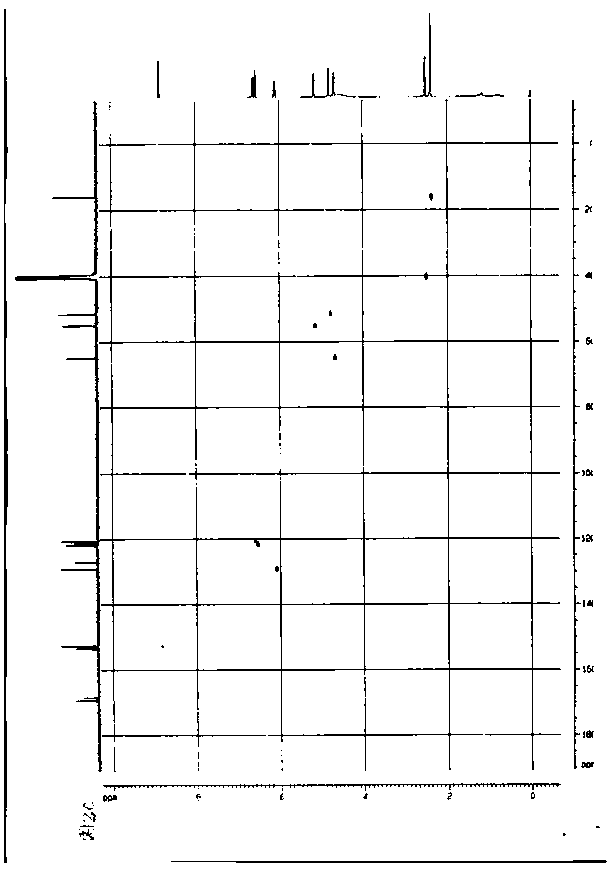
Image
Examples
Embodiment 1
[0038] 7-ATCAδ 3 Preparation of isomers
[0039]Add 0.1mol of 7-ATCA and 350ml of dichloromethane into a 1000ml three-neck flask, add 0.18mol of BSA (N,O-bistrimethylsilylacetamide) under nitrogen protection, stir until all are dissolved and clear, and cool down to 20°C Next, add 0.16 mol of tributylamine, stir for 40 minutes, heat up to 25°C-30°C, stir for reaction, HPLC detects the conversion of isomers, after the reaction is complete, add 350ml of purified water, stir for 10 minutes, let stand, The aqueous layer was separated, and the aqueous layer was washed 3 times with 50 ml of dichloromethane. Cool the water layer to 2-5°C, adjust the pH value to 3.5 with 15% hydrochloric acid, precipitate crystals, stir for 2 hours, filter, wash the filter cake with cold purified water, and filter to dry. Suspend the wet product in 450ml of methanol, heat to dissolve, filter while hot, the filtrate is cooled and crystallized, filtered, the filter cake is washed with acetone, and drie...
Embodiment 2
[0041] 7-ATCAδ 3 Preparation of isomers
[0042] Add 0.1mol of 7-ATCA and 350ml of dichloromethane into a 1000ml three-neck flask, add 0.22mol of BSA (N,O-bistrimethylsilylacetamide) under nitrogen protection, stir until all are dissolved and clear, and cool down to 20°C Next, add 0.2 mol of diethylamine, stir for 30 minutes, heat up to 25°C-30°C, stir for reaction, and detect the conversion of isomers by HPLC. After the reaction is complete, add 350ml of purified water, stir for 10 minutes, and let stand. The aqueous layer was separated, and the aqueous layer was washed 3 times with 50 ml of dichloromethane. Cool the water layer to 2-5°C, adjust the pH value to 4.5 with 10% hydrochloric acid, precipitate crystals, stir for 2 hours, filter, wash the filter cake with cold purified water, and filter to dry. Suspend the wet product in 450ml of methanol, heat to dissolve, filter while it is hot, the filtrate is cooled and crystallized, filtered, the filter cake is washed with ac...
Embodiment 3
[0044] 7-ATCAδ 3 Preparation of isomers
[0045] Add 0.1mol of 7-ATCA and 350ml of dichloromethane into a 1000ml three-necked flask, add 0.2mol of BSA (N,O-bistrimethylsilylacetamide) under nitrogen protection, stir until completely dissolved and clear, and cool down to 20°C Next, then add 0.18mol of triethylamine, stir for 25 minutes, heat up to 25°C-30°C, stir for reaction, HPLC detects the conversion of isomers, after the reaction is complete, add 350ml of purified water, stir for 10 minutes, let stand, The aqueous layer was separated, and the aqueous layer was washed 3 times with 50 ml of dichloromethane. Cool the water layer to 2-5°C, adjust the pH value to 4.0 with 10% hydrochloric acid, precipitate crystals, stir for 2 hours, filter, wash the filter cake with cold purified water, and filter to dry. Suspend the wet product in 450ml of methanol, heat to dissolve, filter while it is hot, the filtrate is cooled and crystallized, filtered, the filter cake is washed with ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com