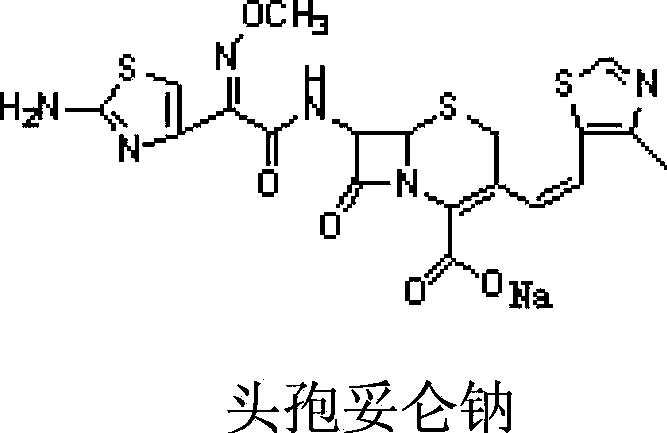
Cefditoren sodium containing-pharmaceutical composition
A technology of cefditoren sodium and its composition, which is applied in the field of pharmaceutical compositions containing cefditoren sodium, can solve the problems of low concentration and poor dissolution effect, and achieve the effects of increasing concentration, improving solubility, and good solubilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 A pharmaceutical composition containing cefditoren sodium, consisting of 0.25 g of cefditoren sodium, 0.15 g of arginine and 0.05 g of citric acid.
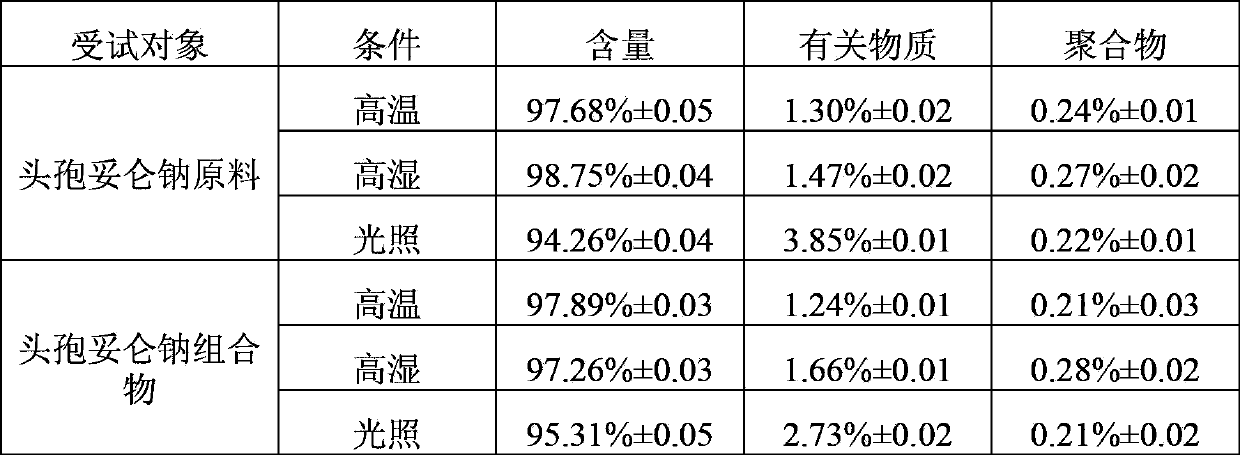
[0021] The stability test of the pharmaceutical composition of this example (with reference to the 2010 "Chinese Pharmacopoeia" guidelines for the stability test of raw materials and pharmaceutical preparations): the pharmaceutical composition of this example and the raw material of cefditoren sodium were subjected to high temperature (60°C) and high temperature respectively. Wet (75%) and light test, measure cefditoren sodium content, related substances and polymer respectively after 10 days, test result is shown in Table 1 (each numerical value in Table 1 is the test number of times n≥6 of corresponding test item obtains respectively mean).
[0022] Table 1
[0023]
[0024] As can be seen from the above-mentioned stability test results, the stability of the pharmaceutical composition of this example is equ...
Embodiment 2
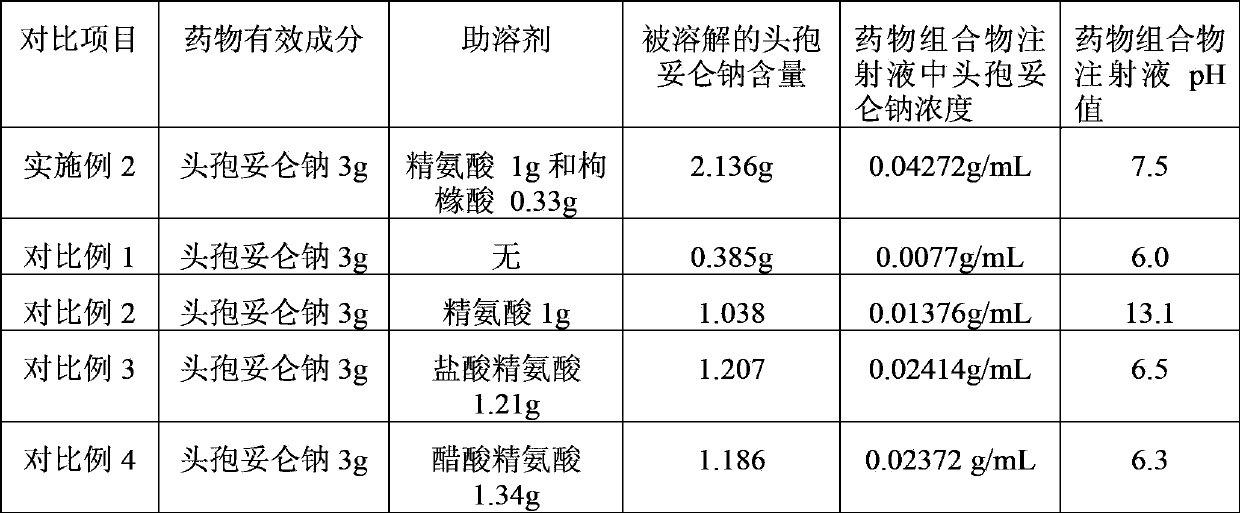
[0028] Example 2 A pharmaceutical composition containing cefditoren sodium, consisting of 3 g of cefditoren sodium, 1 g of arginine and 0.33 g of citric acid.
Embodiment 3
[0034] Example 3 A pharmaceutical composition containing cefditoren sodium, consisting of 0.25 g of cefditoren sodium, 0.4 g of arginine and 0.1 g of tartaric acid.
[0035] Dissolve the pharmaceutical composition of this example with 10 mL of water for injection, the concentration of the active ingredient cefditoren sodium in the injection is 0.025 g / mL, and the pH is 6.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com