Preparation method of high-purity cefditoren pivoxil
A technology for cefditoren pivoxil and cefditoren sodium, which is applied in the preparation of cefditoren pivoxil and the field of compound preparation, can solve the problems of low refining efficiency, low product purity of cefditoren pivoxil, cumbersome industrial production and the like , to achieve the effect of ensuring follow-up treatment, mild and complete reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the preparation of cefditoren pivoxil
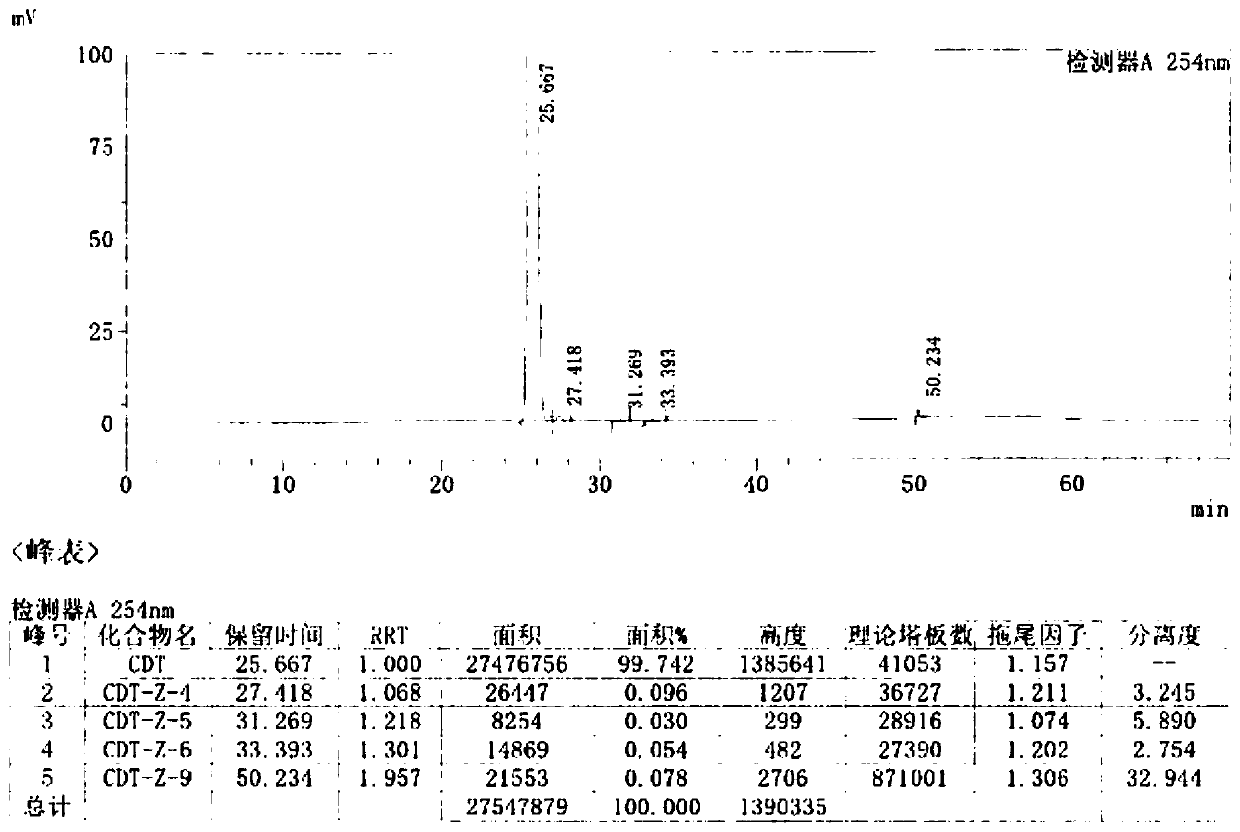
[0051]Control the temperature below 30°C, add 180g of cefditoren sodium to 1.08L of N,N-dimethylformamide, stir until clear, cool down to -35°C, add 78.4g of iodomethyl pivalate dropwise, and control the temperature- 30~-35 ℃ of reaction 1-2 hour, sampling HPLC detects (residual cefditoren sodium is less than 5% is reaction end point), the result shows remaining cefditoren sodium 3.22%, reaction is complete.
[0052] Add 4.4L of isopropyl acetate / water (V / V, 1:1) mixed solvent pre-cooled to 0-5°C to the reaction solution, control the temperature at 0-10°C, stir, extract and separate. Add 0.1% aqueous sodium bicarbonate solution (2.2 L) pre-cooled to 0-5°C to the organic phase, control the temperature at 0-10°C, stir, extract and separate. Add purified water (2.2 L) pre-cooled to 0-5°C to the organic phase, control the temperature at 0-10°C, stir, extract and separate. The organic phase was slowly added to isopropyl...
Embodiment 2
[0053] The mol ratio of embodiment 2, cefditoren sodium and iodomethyl pivalate is: 1:1.1
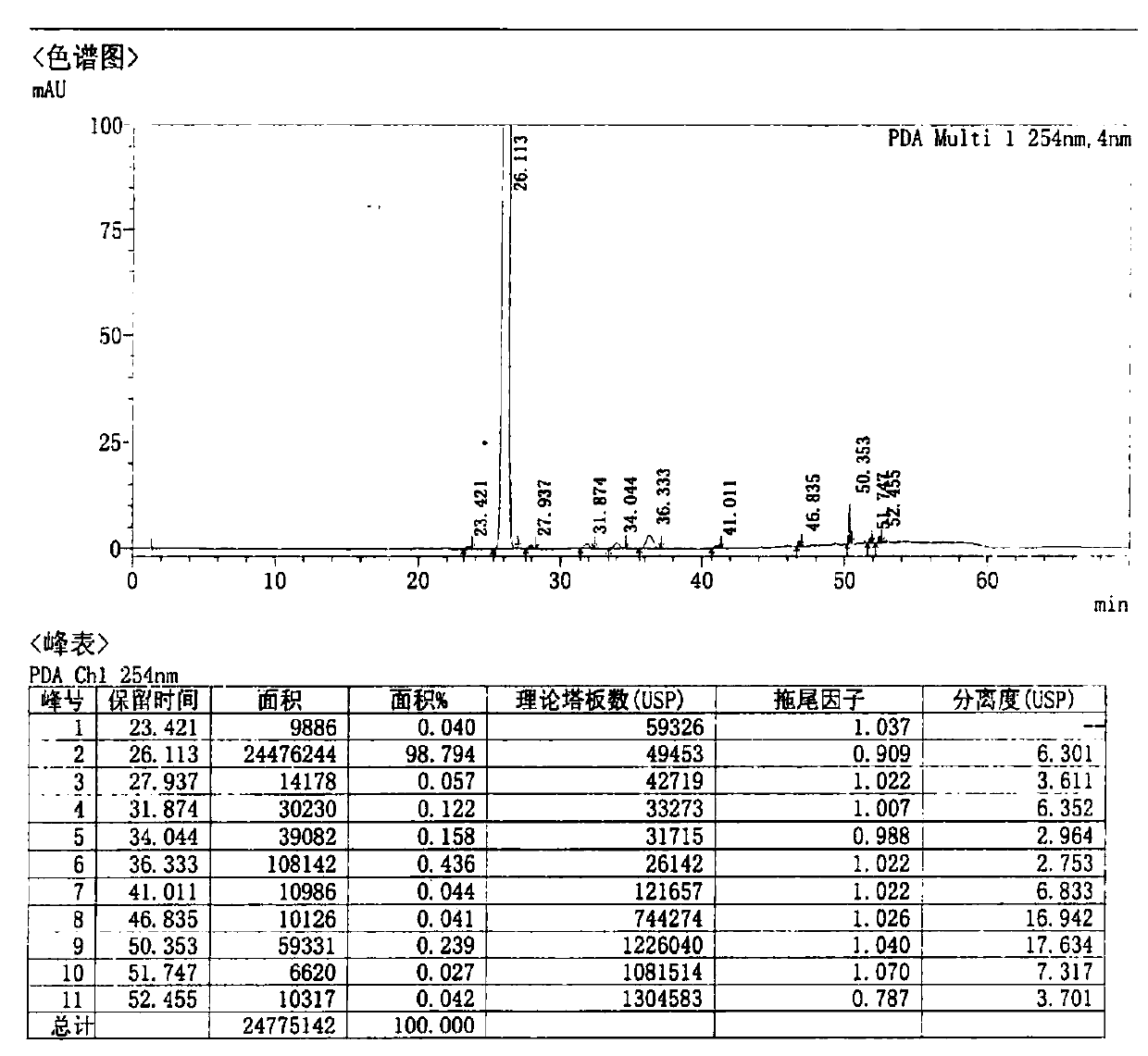
[0054] Control the temperature below 30°C, add 180g of cefditoren sodium to 1.19L of N,N-dimethylformamide, stir until clear, cool down to -35°C, add 78.4g of iodomethyl pivalate dropwise, and control the temperature- 30~-35 ℃ of reaction 1-2 hour, sampling HPLC detects (residual cefditoren sodium is less than 5% is reaction end point), the result shows remaining cefditoren sodium 1.65%, reaction is complete.
[0055] Add 4.4L of isopropyl acetate / water (V / V, 1:1) mixed solvent pre-cooled to 0-5°C to the reaction solution, control the temperature at 0-10°C, stir, extract and separate. Add 0.1% aqueous sodium bicarbonate solution (2.2 L) pre-cooled to 0-5°C to the organic phase, control the temperature at 0-10°C, stir, extract and separate. Add purified water (2.2 L) pre-cooled to 0-5°C to the organic phase, control the temperature at 0-10°C, stir, extract and separate. The organic pha...
Embodiment 3
[0057] Embodiment 3, the reaction temperature of cefditoren pivoxil is changed to -20~-25 ℃
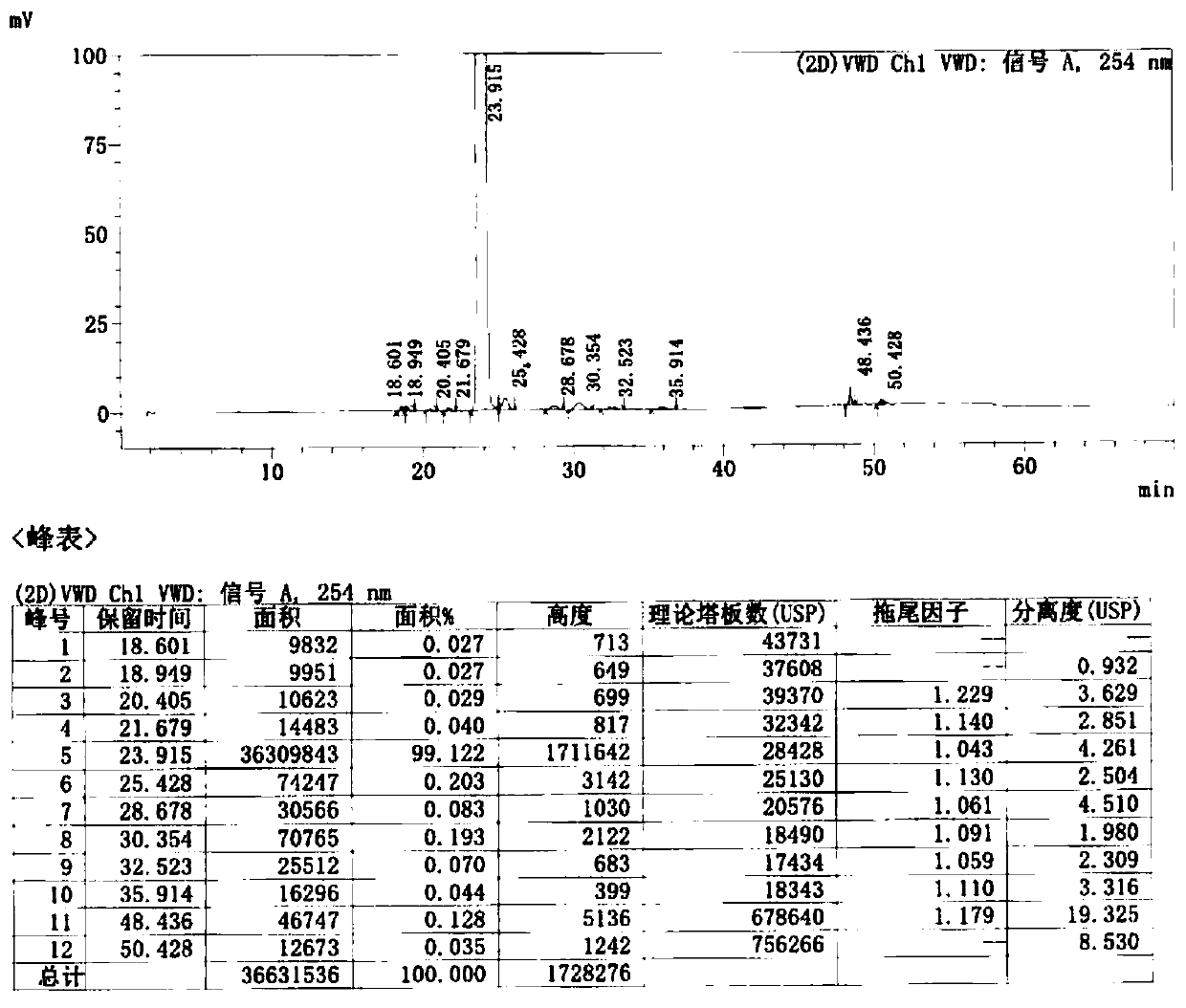
[0058] Control the temperature below 30°C, add 180g of cefditoren sodium to 1.08L of N,N-dimethylformamide, stir until clear, cool down to -25°C, add 78.4g of iodomethyl pivalate dropwise, and control the temperature- 20~-25 ℃ of reaction 1-2 hour, sampling HPLC detects (residual cefditoren sodium is less than 5% is reaction end point), the result shows remaining cefditoren sodium 2.74%, reaction is complete.
[0059] Add 4.4L of isopropyl acetate / water (V / V, 1:1) mixed solvent pre-cooled to 0-5°C to the reaction solution, control the temperature at 0-10°C, stir, extract and separate. Add 0.1% aqueous sodium bicarbonate solution (2.2 L) pre-cooled to 0-5°C to the organic phase, control the temperature at 0-10°C, stir, extract and separate. Add purified water (2.2 L) pre-cooled to 0-5°C to the organic phase, control the temperature at 0-10°C, stir, extract and separate. The organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com