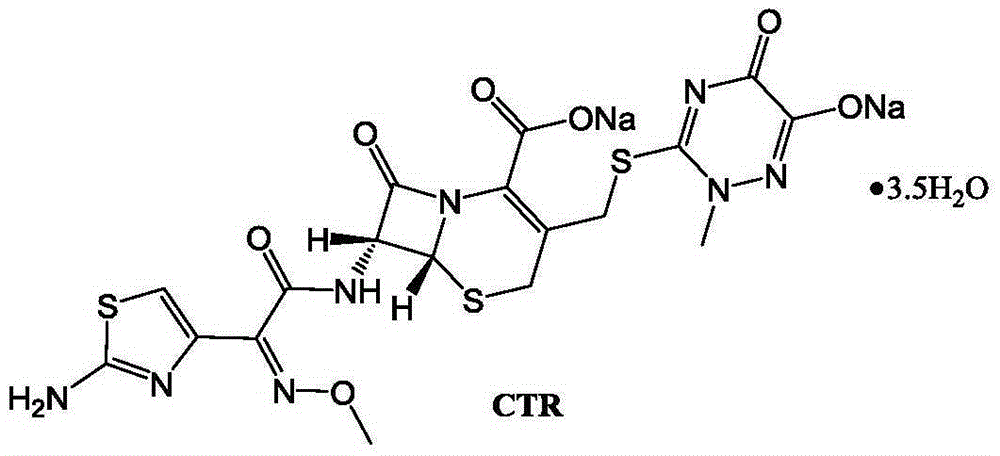
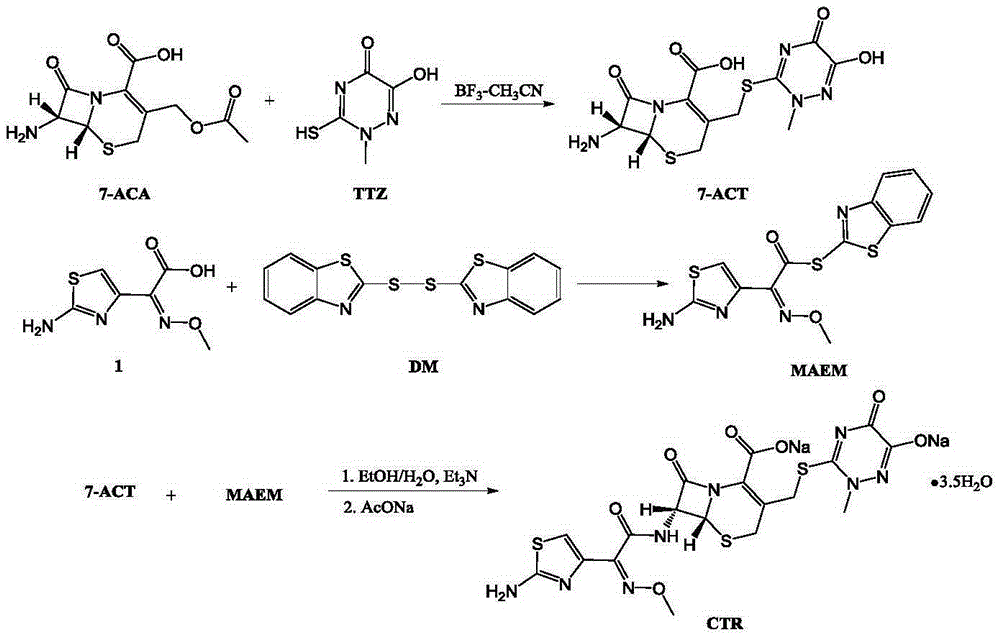
Original-quality ceftriaxone sodium and pharmaceutical preparation thereof
A technology of ceftriaxone sodium and ceftriaxone, which is applied in the field of pharmaceutical preparation, can solve the problems of affecting yield, increasing reaction steps, increasing production costs, etc., and achieves the effects of reducing reaction steps and reducing operation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of 7-ACT
[0021] Add 100 mL of acetonitrile, 40 g (147 mmol) of 7-ACA, and 40.4 g (254 mmol) of TTA into a three-necked flask, stir and cool down to below 10°C, add 150 mL of boron trifluoride-acetonitrile solution [w / w=18%], and heat up to 30°C, react for 30 minutes. Add 300 mL of purified water within 15 minutes, raise the temperature to 10°C-20°C and react for 2 hours, add ammonia water to adjust the pH of the reaction solution to 1.6-2.0, and cool down to 10°C. After filtering, the filter cake was washed with acetonitrile-water and water, and dried to obtain 48g of 7-ACT with a yield of 85.71%.
Embodiment 2
[0022] Embodiment 2: the preparation of CTR
[0023] Add 100mL of acetonitrile, 100mL of dichloromethane, 10g (50mmol) of acetioxamic acid, 15.3g (150mmol) of triethylamine into a three-neck flask, stir and cool down to -5°C-0°C, and slowly dropwise add 5.2g (55mmol) of methyl chloroformate , Stir at -5°C-0°C for 1 h, rise to room temperature and stir for 4 h, add 18.6 g (50 mmol) of 7-ACT, and continue stirring for 6 h. After the reaction is complete, add 200mL of purified water, stir for 10min, let stand to separate layers, discard the organic phase, add the water phase to a three-necked flask, add 9g of sodium acetate solid, stir to dissolve, slowly add about 200mL of acetone dropwise at room temperature, and cool down to 0- At 5°C, continue to add 600 mL of acetone dropwise. After the dropwise addition, grow the crystal for 2 hours, filter, wash the filter cake with 100 mL of acetone, and dry to obtain 27.1 g of ceftriaxone sodium. Yield 82%, purity 99.87%.
Embodiment 3
[0024] Embodiment 3: the preparation of CTR
[0025] Add 100mL of acetonitrile, 100mL of dichloromethane, 10g (50mmol) of amothioxamic acid, 15.3g (150mmol) of triethylamine into a three-necked flask, stir and cool down to -5°C-0°C, and slowly dropwise add 5.45g (50mmol) of ethyl chloroformate , Stir at -5°C-0°C for 1 h, rise to room temperature and stir for 4 h, add 18.6 g (50 mmol) of 7-ACT, and continue stirring for 6 h. After the reaction is complete, add 200mL of purified water, stir for 10min, let stand to separate layers, discard the organic phase, add the water phase to a three-necked flask, add 9g of sodium acetate solid, stir to dissolve, slowly add about 200mL of acetone dropwise at room temperature, and cool down to 0- At 5°C, continue to add 600 mL of acetone dropwise. After the dropwise addition, grow the crystal for 2 hours, filter, wash the filter cake with 100 mL of acetone, and dry to obtain 27.5 g of ceftriaxone sodium. Yield 83.14%, purity 99.85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com