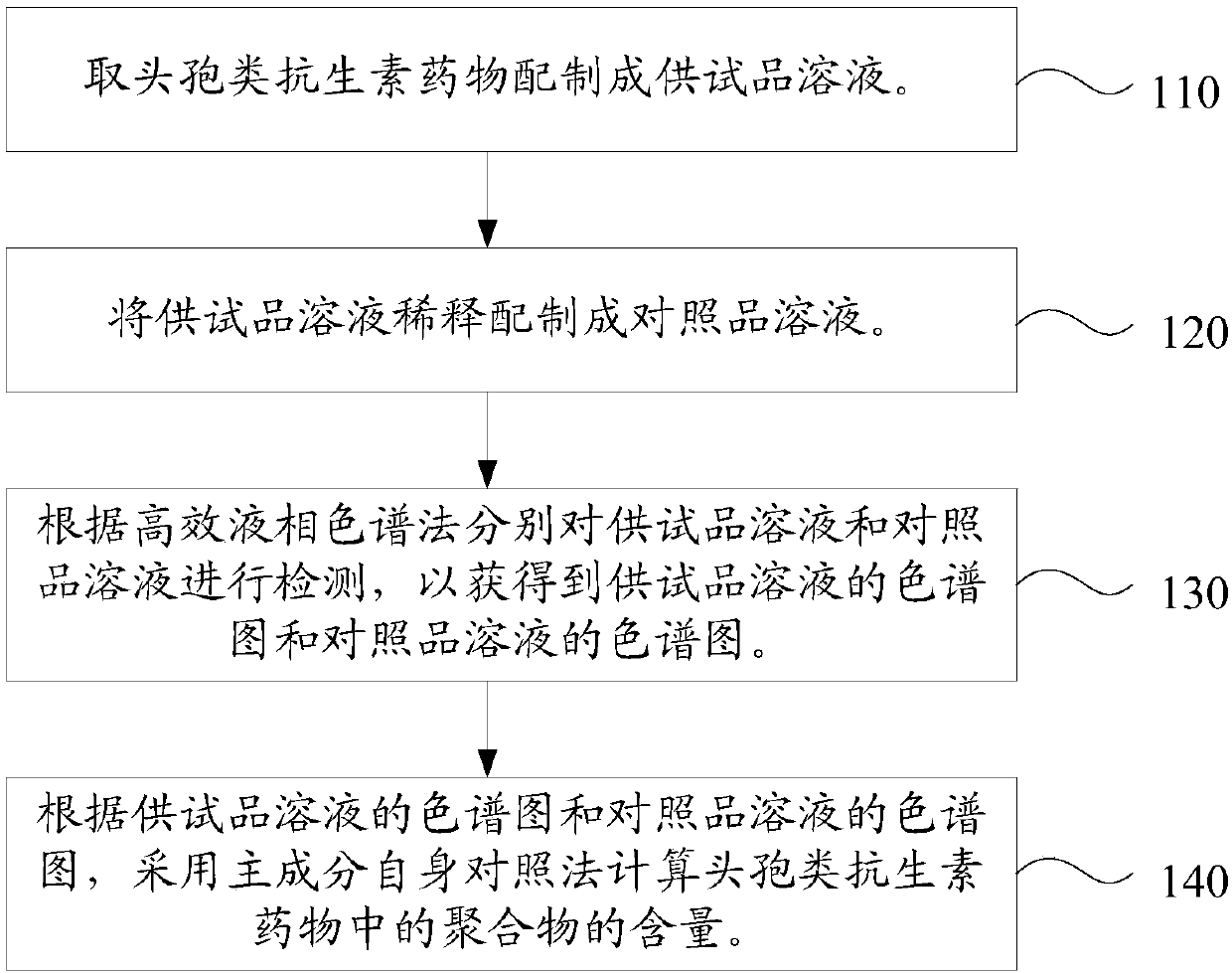
Content determination method of polymer in cephalosporin antibiotic drug
A method of determination, antibiotic technology, applied in the field of polymer content determination in cephalosporin antibiotics, can solve the problem of low polymer accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The content assay method of polymer in the cefathiamidine raw material of the present embodiment is as follows:
[0058] (1) Accurately weigh 20 mg of cefathiamidine raw material, place it in a 20 mL measuring bottle, dissolve it in water, and use it as the test solution.
[0059] (2) Precisely pipette 1 mL of the test solution, dilute it with water to 100 mL, and use it as a control solution.
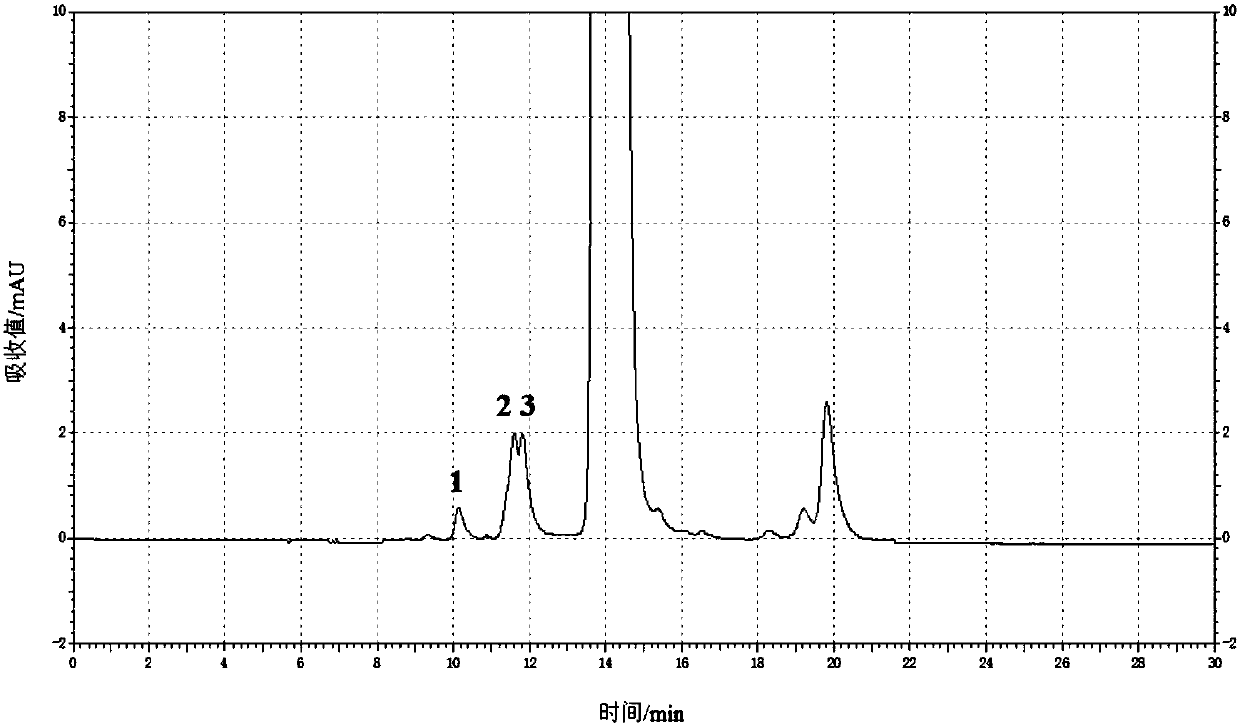
[0060] (3) according to high performance liquid chromatography, need testing solution and contrast solution are detected respectively, to obtain the chromatogram of need testing solution (such as figure 2 Shown) and the chromatogram of contrast solution, wherein, chromatographic condition is: chromatographic column is TSK-GELG2000SW XL Gel chromatographic column (7.8mm ID × 30cm, 5μm), the filler is spherical silica gel, and the pore diameter of the filler is The mobile phase is a mixture of ammonium acetate aqueous solution and acetonitrile, the volume ratio of ammonium ace...
Embodiment 2
[0080] The content assay method of polymer in the cefathiamidine raw material of the present embodiment is as follows:
[0081] (1) Accurately weigh 20 mg of cefathiamidine raw material, place it in a 20 ml measuring bottle, dissolve it in water, and use it as the test solution.
[0082] (2) Accurately pipette 1ml of the test solution and dilute it with water to 100ml as a control solution.
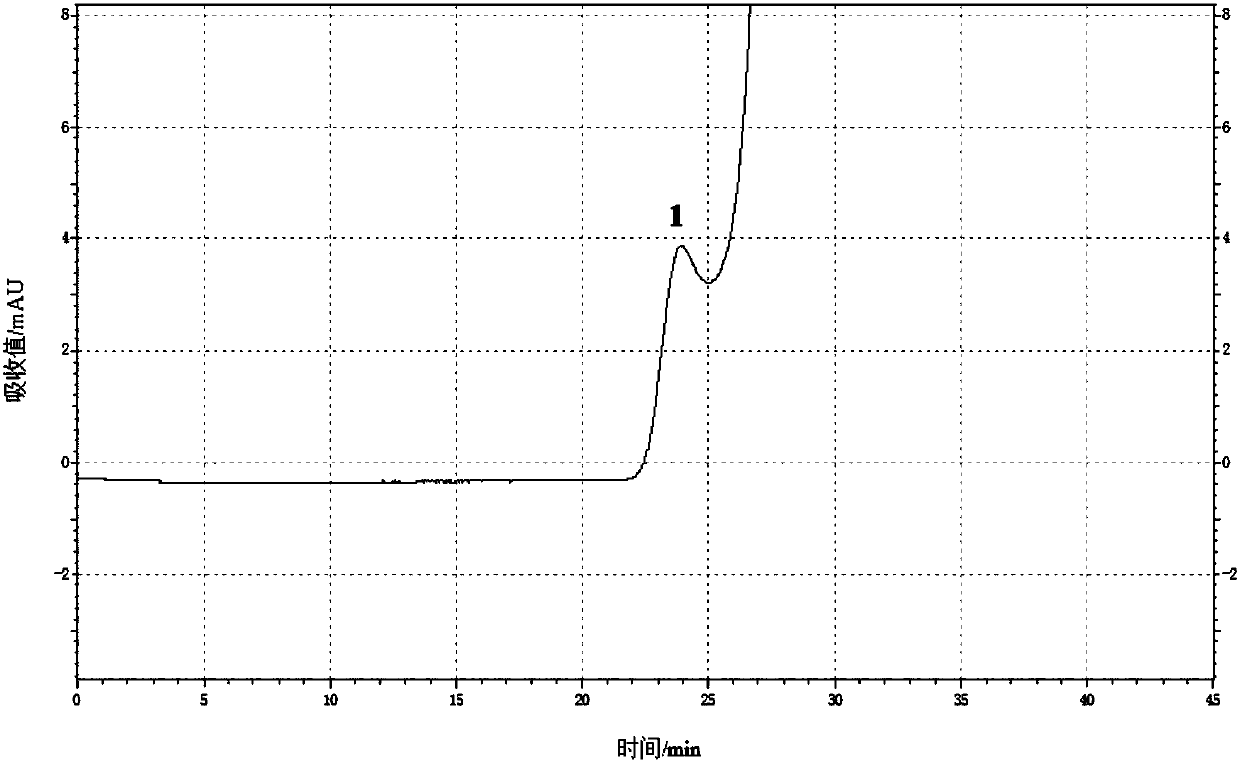
[0083] (3) according to high performance liquid chromatography, need testing solution and contrast solution are detected respectively, to obtain the chromatogram of need testing solution (such as Figure 4 Shown) and the chromatogram of contrast solution, wherein, chromatographic condition is: chromatographic column is TSK-GELG2000SW XL Gel chromatographic column (7.8mm ID × 30cm, 5μm), the filler is spherical silica gel, and the pore diameter of the filler is The mobile phase is a mixture of ammonium acetate aqueous solution and acetonitrile, the volume ratio of ammonium acetate aqueo...
Embodiment 3
[0087] The assay method of polymer in the cefathiamidine for injection of the present embodiment is as follows:
[0088] (1) Accurately weigh 20 mg of cefathiamidine for injection, place it in a 20 mL measuring bottle, dissolve it in water, and use it as the test solution.
[0089] (2) Precisely pipette 1 mL of the test solution, dilute it with water to 100 mL, and use it as a control solution.
[0090] (3) according to high performance liquid chromatography, need testing solution and contrast solution are detected respectively, to obtain the chromatogram of need testing solution (such as Figure 5 Shown) and the chromatogram of contrast solution, wherein, chromatographic condition is: chromatographic column is TSK-GELG2000SW XL Gel chromatographic column (7.8mm ID × 30cm, 5μm), the filler is spherical silica gel, and the pore diameter of the filler is The mobile phase is a mixture of ammonium acetate aqueous solution and acetonitrile, the volume ratio of ammonium acetate a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com