Kidney transplant donor-specific urine source cell and its DNA preparation method and application thereof
A specific technology for kidney transplantation, which is applied in the field of biomedicine, can solve the problems of obtaining six-site or eight-site genotype information, the lack of storage time of donor DNA samples, and the inability to accurately determine donor HLA information, etc., to achieve the goal of obtaining The method is completely non-invasive, the acquisition method is safe and non-invasive, and the effect of improving the success rate of sequencing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
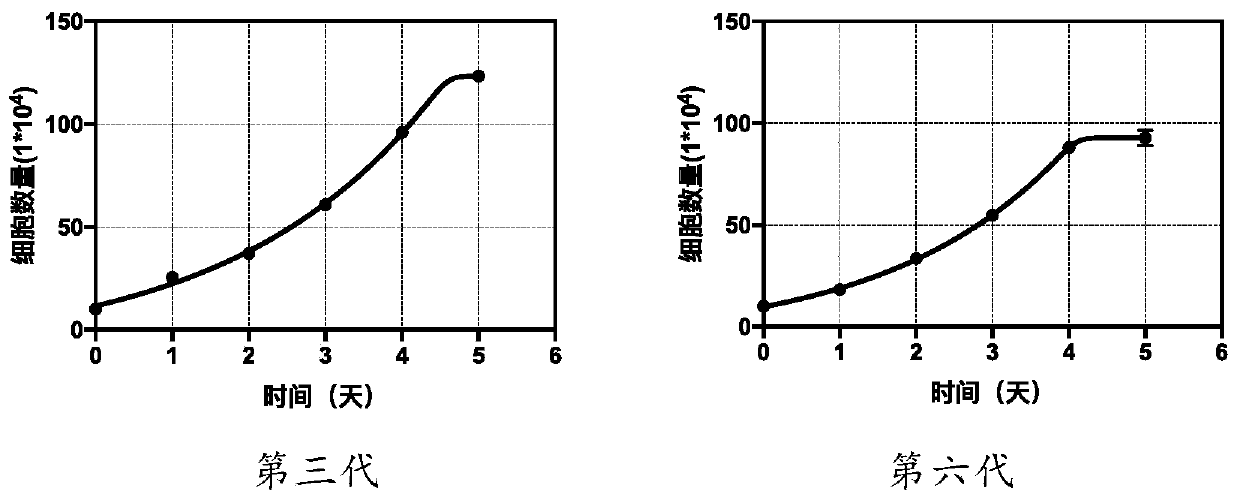
[0049] Example 1 Preparation of donor-specific urine-derived cells
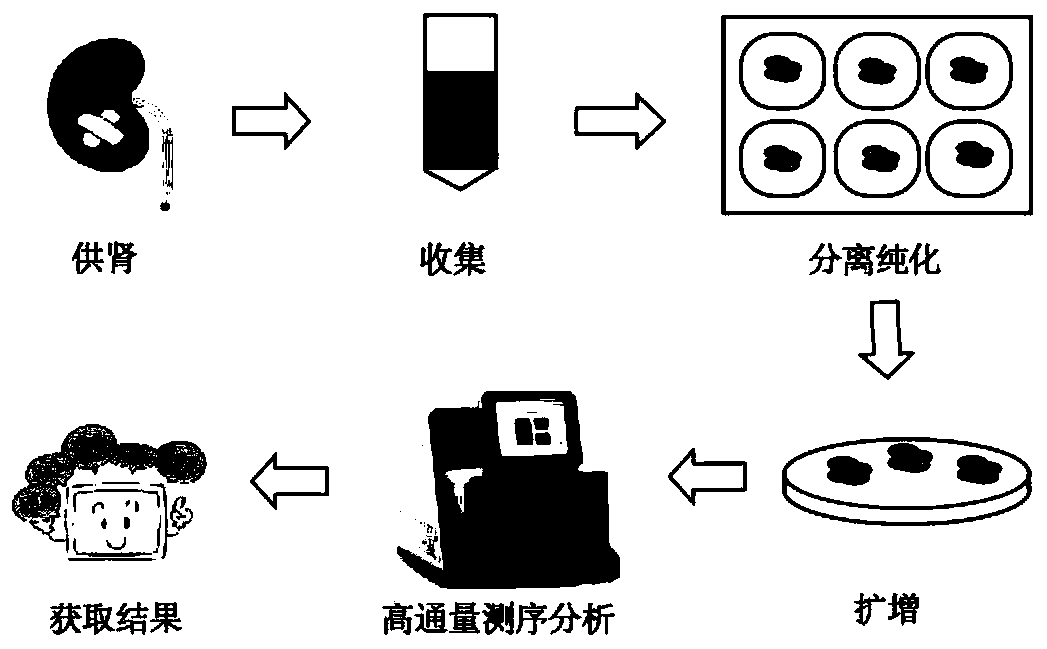
[0050] 1.1 The separation and preparation of donor-specific urine-derived cells, the method is as follows:
[0051] 1) Take 150-200mL of clean middle urine from the patient (recipient);
[0052] 2) Divide the urine into 50mL centrifuge tubes and centrifuge at 400g for 10min;
[0053] 3) Use an aspirator to slowly suck off the supernatant, leaving about 3-5mL of urine;
[0054] 4) Add about 20-30mL of phosphate-buffered saline (PBS) containing penicillin-streptomycin mixture, mix gently by pipetting, and centrifuge again at 400g for 10min;
[0055] 5) Use an aspirator to slowly suck off the supernatant until the remaining liquid is less than 1mL;
[0056] 6) Add 1 mL of urine-derived cell culture medium, resuspend the remaining precipitate, and gently pipette several times;
[0057] 7) The above-mentioned cell suspension was uniformly inoculated in a 24-well plate coated with 0.1% gelatin solution (Gelatin...
Embodiment 2
[0080] Example 2 Using donor-specific urine-derived cells to detect donor HLA high-resolution typing
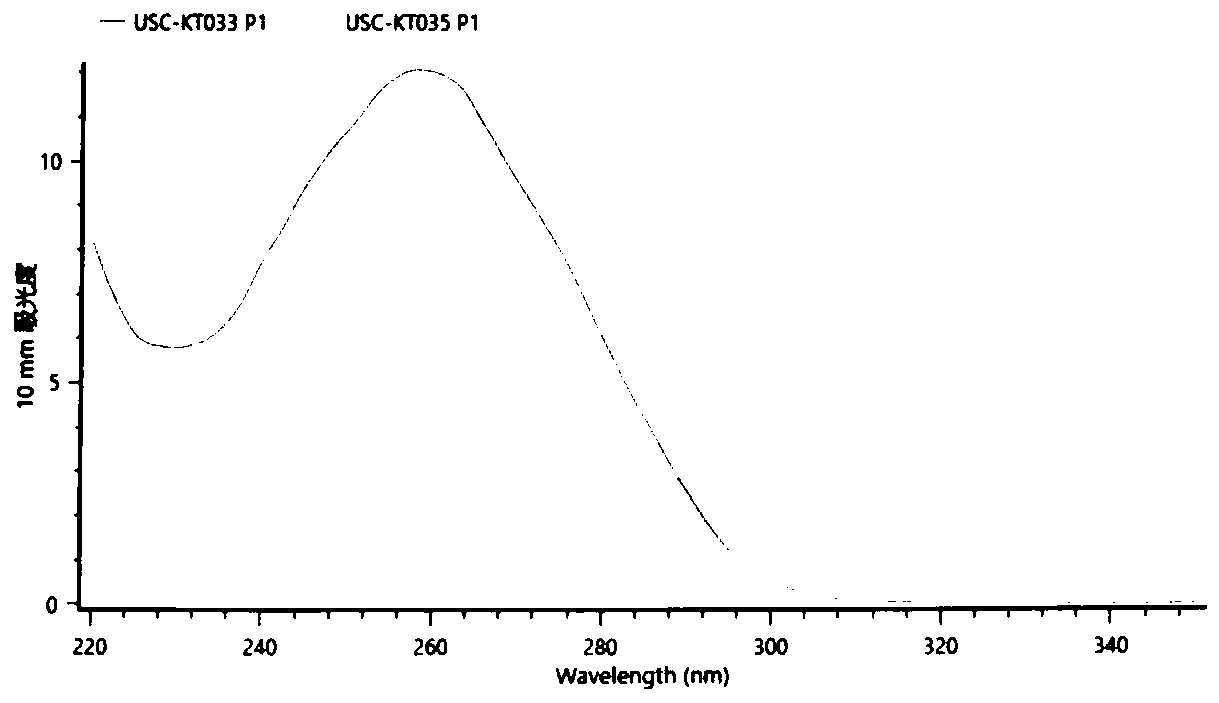
[0081] 2.1 Extraction of genomic DNA from donor-specific urine-derived cells (using Tiangen-genomic DNA Kit)
[0082] 1) After the cells proliferate to 1*10 6 Digest each cell with 0.25% EDTA trypsin at 37°C for about 3-5min;
[0083] 2) Add an equal volume of basal medium containing 10% serum to stop the digestion, and gently blow the cells to make them fall off the bottom of the dish;
[0084]3) Transfer the cell suspension to a 15mL centrifuge tube and centrifuge at 200g for 5min;
[0085] 4) Add 1ml of phosphate buffered saline (PBS), resuspend the cells, centrifuge at 10000rpm (~11200g) for 1min, pour off the supernatant, add 200ul of buffer A, shake until completely suspended;
[0086] 5) Add 4 ul RNase A (100 mg / ml), vortex for 15 s, and place at room temperature for 5 min; add 20 ul proteinase K (Proteinase K), and mix well.
[0087] 6) Add 200ul of buffer solutio...
Embodiment 3
[0107] Example 3 Application of donor-specific urine-derived cells to detect high-resolution typing of donor HLA after kidney transplantation in adult kidney transplantation
[0108] 3.1 Using the above methods to detect the results of HLA high-resolution typing after kidney transplantation in adults
[0109] Patient 007, female, 42 years old, donor age 26 years old, nine months after kidney transplantation. Table 3 shows the HLA high-resolution typing results of urine-derived cells and urine sediment after adult kidney transplantation detected in the examples of the present invention. It can be seen that the HLA high-resolution typing results of the recipient itself are consistent with the sequencing results of the recipient's urine sediment. The HLA sequencing results of the recipient's urine-separated cells were consistent with the donor's HLA high-resolution typing results. This method is suitable for adult kidney transplant recipients, and can obtain donor-specific HLA h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com